01 Jan 2019
Author: Sumithra Urs, PhD | Scientist, Scientific Development
Date: January 2019
Acute myeloid leukemia (AML) is the most common hematologic malignancy in adults with a 5-year survival rate of ~25% following diagnosis.[1] While two-thirds of AML patients treated with standard high dose chemotherapy achieve remission, 50% of patients relapse after remission. The majority of relapses occur within two to three years of initial treatment, and every patient carries the risk of relapse due to the molecular heterogeneity of the disease.[2] This has created an impetus to explore novel therapeutic approaches; in particular, immune-based therapies, since AML cells express both major histocompatibility complex (MHC) classes I and class II which makes them susceptible targets of innate and adaptive immune responses.[3]
In our April 2017 model spotlight, we presented data on preclinical model development of the C1498-Luc-mCherry systemic acute myeloid leukemia model in C57BL/6 mice. The line is highly aggressive as a disseminated model, with a median tumor doubling time of 1.3 days based on bioluminescence imaging (BLI) and a median overall survival of ~23 days. In continued efforts to expand the model for immuno-oncology applications, we present here data on response to immune checkpoint blockade.
C1498-Luc-mCherry Tumor Cell Distribution
Following IV implantation of cells, we find that solid tumor masses will develop in a number of tissues including the ovaries, liver, and spine. To understand the distribution of C1498-Luc-mCherry tumor cells in the model, flow cytometry was performed on spleen, bone marrow, and tumors found on/around the ovaries. Samples were collected from five C57BL/6 mice 21 days post implant of C1498-Luc-mCherry. As the line is a myeloid malignancy it’s unsurprising that 97% of the cells analyzed were CD45+ in the tumors around the ovary. We determined, by examination of mCherry, that very few tumor cells were present in the spleen (~2%) and bone marrow (6%) while almost 78% of the CD45+ cells in the ovarian-based tumors were mCherry+ (Fig. 1A). Histopathological evaluation of H&E stained sections of ovarian-based tumors confirmed the presence of homogeneous populations of neoplastic cells with similar morphology. Tumor masses were composed of moderately pleomorphic tumor cells with round to oblong nuclei, and scant cytoplasm with a high number of mitotic figures (Fig. 1B).
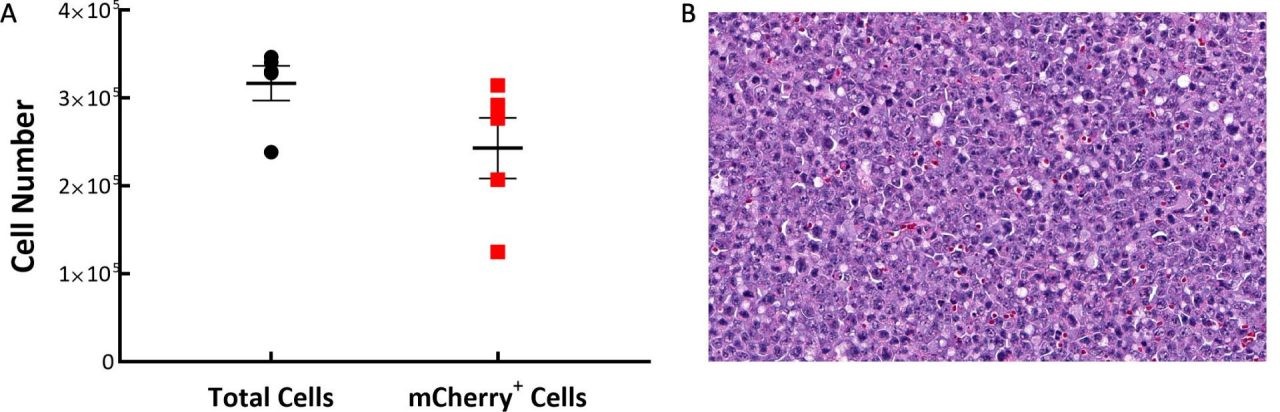
Fig. 1: C1498-Luc-mCherry tumor composition in C57BL/6 mice. A: Tumors are mostly composed of mCherry+ cells. B: A representative H&E-stained section showing neoplastic cells with pleomorphic nuclei and multiple mitotic figures, 20X original magnification.
C1498-Luc-mCherry Response to Cyclophosphamide
To confirm the sensitivity of the C1498-Luc-mCherry tumor model to cyclophosphamide, we tested the broad spectrum chemotherapeutic agent used to treat multiple cancers, including leukemia, in mice with established disease. Treatment with 100mg/kg resulted in complete regression and 90% (9/10) tumor free survivors (TFS, Fig. 2). Although we saw remission, cyclophosphamide treated animals showed enlarged ovaries and ovarian horn, discolored spleen and fluid in the peritoneum at necropsy. Cytotoxicity to cyclophosphamide treatment in patients is known to have several adverse side effects including bone-marrow suppression, hemorrhagic cystitis, increased susceptibility to infections, infertility and the carcinogenic risk of developing other malignancies. Severe toxicity of chemotherapy has helped steer the way to explore alternate therapeutic approaches such as immunotherapies.
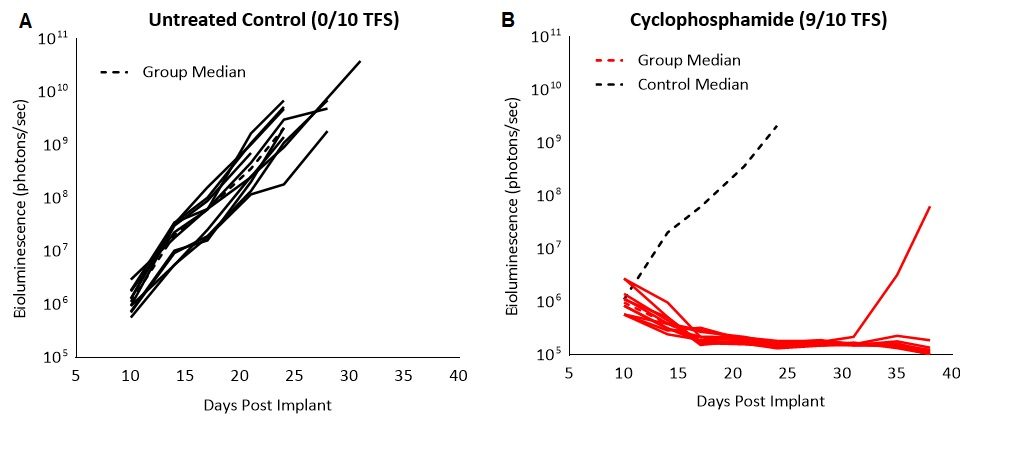
Fig. 2: C1498-Luc-mCherry response to cyclophosphamide treatment in C57BL/6 mice. A and B: Bioluminescence signal from each individual mouse over time. Dotted black line indicates median signal of untreated control.
C1498-Luc-mCherry Response to Blockade of Immune Checkpoints
Previously published data showed that the C1498 model expressed PD-L1 in vivo and blockade with anti-PD-L1 antibody improved survival.[4] In our study, we evaluated the response of the C1498-Luc-mCherry model to anti-mPD-1 and anti-mPD-L1 immune checkpoint inhibitors in C57BL/6 mice with established disease. Treatment with anti-mPD-1 or anti-mPD-L1 resulted in modest tumor growth delay (3 and 5.2 days, respectively, excluding the outlier in anti-PD-L1 group) and increased life span (25% and 16.7%, respectively, Fig. 3 and 4). Treatment related adverse effects on body weight were not observed, however, accumulation of ascites resulted in abdominal distention as disease progressed in all groups. Necropsy showed masses in the ovaries, mottled liver and enlarged lymph nodes.
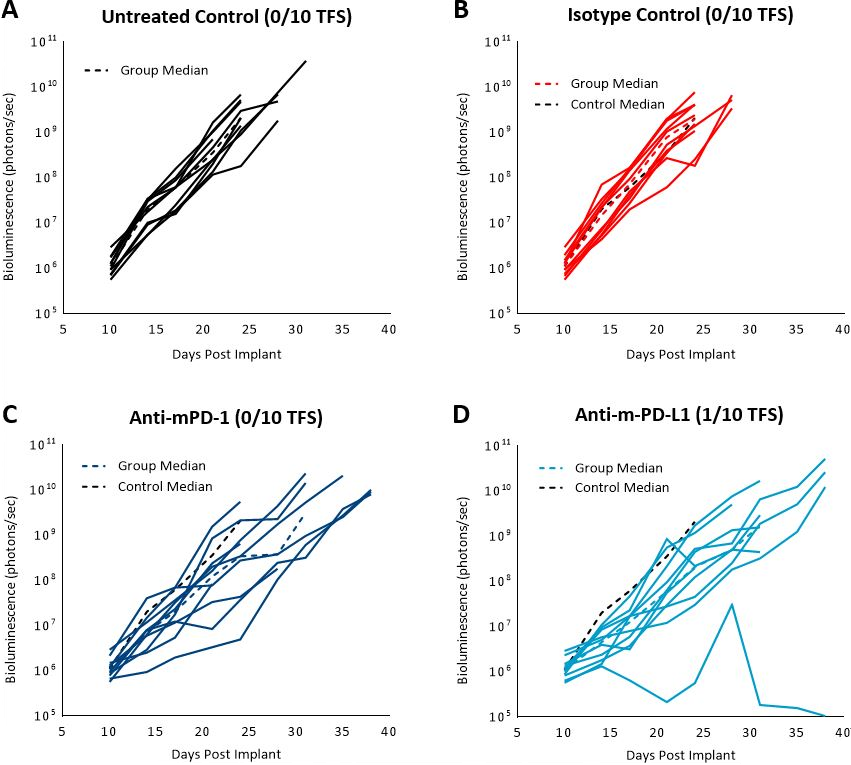
Fig. 3: C1498-Luc-mCherry response to checkpoint inhibitors in C57BL/6 mice. A, B, C and D: Bioluminescence signal from each individual mouse over time. Dotted black line indicates median signal of untreated control. Colored dotted lines indicate median signal of each group.
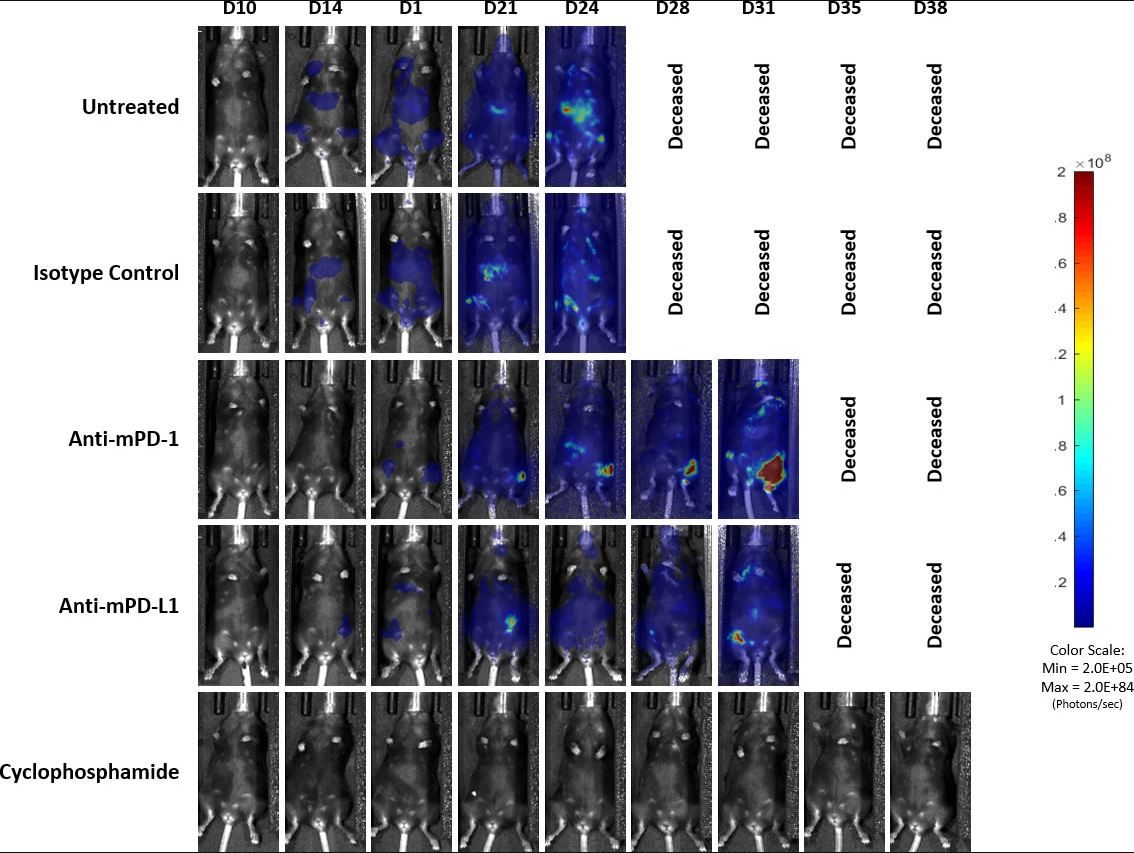
Fig. 4: Representative images of BLI signal in C1498-Luc-mCherry disseminated model in C57BL/6 mice
Preliminary clinical trial reports of AML immunotherapy with PD-1/PD-L1 blockade has indicated that successful response to the immune-modulating agents, as in many forms of cancers, is seen only in particular subgroups of AML patients.[5] In general, monotherapy with immune checkpoint inhibitors for AML is being considered ineffective for patients with more than minimal disease because of rapid disease progression, small window for response, and tumor heterogeneity. Therefore, clinical focus is shifting towards combination therapies. As the response to anti-mPD-1 and anti-mPD-L1 is minimal in this model, it provides ample opportunity for preclinical investigation into novel drug combinations with these immune modulatory agents.
Contact us today to discuss whether the C1498-Luc-mCherry model is right for your next AML study.
