01 Aug 2021
Author: Patrick Allison, PhD | Senior Scientist, Scientific Development
Date: August 2021
Introduction
Multiple myeloma is a hematological malignancy that arises from antibody-producing plasma cells that is rarely detected prior to symptomatic disease onset. Multiple myeloma was the cause of nearly 100,000 deaths worldwide in 20181 and it is estimated that in 2021, approximately 35,000 new cases of multiple myeloma will be diagnosed and cause 12,500 deaths United States2.
Multiple myeloma takes up residence and produces lytic lesions in the bone marrow – in addition to death from tumor progression, multiple myeloma lesions result in bone pain, fracture, anemia, and kidney dysfunction as a result of paraprotein production by malignant plasma cells that can cause damage to other organs.
There are several clinically approved therapeutics for the treatment of multiple myeloma with diverse mechanisms of action, including cyclophosphamide (DNA alkylating agent), bortezomib and carfilzomib (proteasome degradation blockade), and doxorubicin (DNA intercalation). There are also approved therapies that target aspects of the immune system, including daratumumab and isatuximab (CD38 targeting monoclonal antibodies)3. Many of these therapies are either approved or under clinical investigation to be used in combination with one another.
To understand the potential anti-tumor effects of novel therapeutics in combination with clinical standards of care, selection of the most appropriate preclinical tumor model is crucial. Human xenograft and mouse tumor models represent such a preclinical platform for evaluating novel test agents. At Labcorp Drug Development (formerly Covance Laboratories), we have a wide selection of tumor models for evaluating the efficacy and mechanism of action of test agents against multiple myeloma. MM1.S and NCI-H929 human multiple myeloma are two commonly used tumor cell lines grown in immunodeficient mice permissive of hosting human cells. 5TGM-1 is a multiple myeloma tumor line that was originally derived from mice, representing a syngeneic tumor model in which the host mouse has a fully competent immune system to evaluate immune-modulating agents. We have enabled these lines to express luciferase, an enzyme that produces bioluminescence in the presence of the substrate luciferin, in which light output can be measured as an indication of tumor burden and disease progression via bioluminescence imaging (BLI). These models are implanted into mice intravenously, disseminating the disease throughout the animal and producing bone lesions in a manner similar to clinical disease progression.
In these studies, we evaluate each model’s response to clinical standards of care, to characterize it as responsive (or refractory), so that our clients can select the most appropriate model for their program and identify opportunities for combination studies.
MM1.S Human multiple myeloma
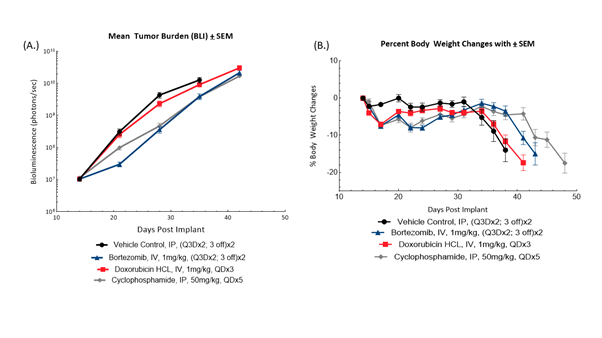
Fig. 1: Longitudinal BLI (A) and bodyweight changes of female SCID Beige mice (B) bearing disseminated MM1.S-Luc-Neo multiple myeloma tumors in response to bortezomib, doxorubicin and cyclophosphamide (n=10/group).
MM1.S-Luc-Neo human multiple myeloma cells were implanted intravenously into female SCID Beige mice (Jackson Laboratories, Bar Harbor Maine, USA). Animals were subjected to BLI to quantify tumor burden in photons/second and stratified into treatment groups to equalize group disease burden prior to treatment (Figure 1). Treatment with vehicle control resulted in uncontrolled disease progression as monitored by BLI, progressive bodyweight loss typical of the model, and all mice exiting the study by 31 days following tumor implant. Treatment with bortezomib resulted in modest anti-tumor activity as evidenced by BLI %T/C on Day 35 of 36% compared to control and a 31% increase in time to progression. Administration of cyclophosphamide conferred a similar anti-tumor effect, resulting in %T/C compared to control of 38% on Day 35 and a 25% increase in time to progression. Administration of doxorubicin HCL did not provide meaningful anti-tumor activity (%T/C of 85% of control on Day 35, and 6% increase time to progression) or survival benefit. Treatment with bortezomib or cyclophosphamide resulted in delay of disease-related bodyweight loss. Taken together, MM1.S-Luc-Neo human multiple myeloma provides a model appropriate for evaluating potential synergies of cyclophosphamide or bortezomib in combination with novel agents that aim to improve outcomes paired with existing therapies. For additional data in this model, see our previous model spotlight[P1] .
NCI-H929 Human multiple myeloma
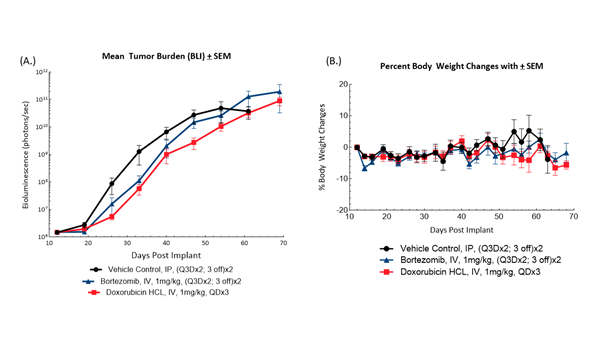
Fig. 2: BLI measurements (A) of and bodyweight changes (B) in female NSG mice bearing disseminated NCI-H929-Luc-mCh-Puro human multiple myeloma administered bortezomib or doxorubicin HCL (n=10/group).
Female NSG mice (Jackson Laboratories, Bar Harbor, Maine, USA) were intravenously implanted with NCI-H929-luc-mCh-Puro human multiple myeloma cells to induce disseminated disease. Mice were subjected to BLI to detect tumor burden and sort them into treatment groups prior to administration of bortezomib or doxorubicin HCL (Figure 2). Treatment with either agent showed early but modest suppression of tumor growth by BLI. Treatment with bortezomib resulted in %T/C compared to control of 31% on Day 40 and a 22% increase in time to progression. Administration of doxorubicin HCL resulted in %T/C compared to control of 59% and a 12% increase in time to progression. Bodyweight loss was moderate and typical of the model. This demonstrates an opportunity for evaluating combination treatment to improve response to these clinical standards of care in the NCI-H929-Luc-mCh-Puro model.
5TGM-1 Murine model of multiple myeloma
5TGM1 murine multiple myeloma arose spontaneously in aging C57BL/KaLwRij mice4and was enabled with the luciferase enzyme to allow for BLI monitoring of disease progression. This model represents a syngeneic multiple myeloma model in mice with intact immune systems that can be exploited to determine the anti-tumor activity of immune-modulatory agents.
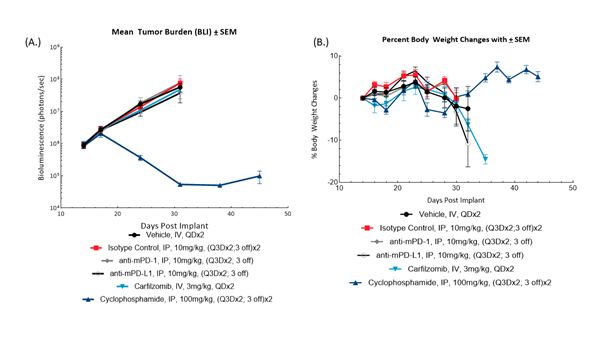
Fig. 3: BLI monitoring for 5TGM1-luc tumor burden (A) and bodyweight changes (B) in female C57BL/KaLwRij mice administered checkpoint-inhibiting antibodies, carfilzomib or cyclophosphamide.
Female C57BL/KaLwRij mice (Envigo, The Netherlands) were intravenously implanted with 5TGM1-Luc mouse multiple myeloma cells and subjected to BLI to sort animals by tumor burden for treatment. Animals were administered isotype control (clone 2A3), anti-mPD-1 (clone RMP1-14), anti-mPD-L1 (clone 10F.9G2), carfilzomib or cyclophosphamide (Figure 3). All antibodies were sourced from BioXCell (Lebanon, New Hampshire, USA). BLI monitoring revealed treatment with anti-mPD-1 or anti-mPD-L1 did not result in anti-tumor activity, and disease-related bodyweight loss was not abrogated. This represents an opportunity for evaluating agents that sensitize the immune system to checkpoint therapy. Administration of cyclophosphamide resulted in meaningful and durable anti-tumor activity resulting in 4 partial regressions and 3 complete responses; further evaluation of cyclophosphamide response is required to evaluate appropriate combination doses.
Taken together, we have demonstrated the robust and powerful human xenograft and mouse tumor models available at Labcorp and how they can be utilized to compare against or improve response to clinical standards of care in these multiple myeloma models. Contact our scientists to learn more about these and other hematologic models and how we can apply these to advance your oncology pipeline.
