01 Mar 2019
Author: Sheri Barnes, PhD | Director, Scientific Development &Maryland Franklin, PhD | Vice President, Scientific Development
Date: March 2019
Colorectal cancer is the fourth most common cancer diagnosed in the United States. Colorectal cancer represents the third leading cause of cancer-related deaths in women and the second in men. In 2019, over 145,000 estimated new cases of colorectal cancer in the United States will be diagnosed and more than 51,000 patient deaths will occur. Prevention and early detection initiatives over the last several decades, together with improved treatment options, have resulted in reductions in colorectal cancer diagnoses and deaths. These measures have also increased the five-year overall survival rate to 64.9%, but survival drops precipitously for those patients whose cancer is not detected early.[1] For this reason, the development of new treatments for colorectal cancer is a continual need.
The advent of immunotherapy has necessitated syngeneic mouse tumor models with desirable growth kinetics and response to immunomodulatory agents to further advance development of immuno-oncology treatments. One of these colon adenocarcinoma models, MC38, has been characterized by Labcorp to support development of these agents. MC38 was isolated from a colon tumor in a C57BL/6 mouse following long term exposure to the carcinogen DMH (1,2-dimethylhydrazine dihydrochloride).[2] As described below, MC38 has a favorable response profile to immunomodulatory antibodies suggesting a tumor microenvironment amendable to immune activation. Thus, MC38 is well positioned to be a powerful immuno-oncology model with significant utility in drug development
MC38 Tumors Following Checkpoint Inhibitor Therapy
The in vivo doubling time of subcutaneous MC38 tumors is ~4 days, a moderate growth rate which can facilitate up to a three-week dosing window for test agents to elicit their anti-tumor activity. The model was used in a study to evaluate response to commonly utilized checkpoint inhibitor antibodies. Figure 1 demonstrates mean tumor volumes (A) and individual tumor volumes (B-F) of untreated control tumors compared to those treated with isotype control, anti-mCTLA-4, anti-mPD-L1, or anti-mPD-1. Dosing with all test agents began once tumors were established (~100mm3). Anti-mPD-L1 and anti-mPD-1 demonstrated the most meaningful anti-tumor activities, of the three checkpoint inhibitors, with approximately 6 and 8 days of tumor growth delay on day 22, 40% and 50% putative responders, and an increased time to progression of 32 and 29 days, respectively compared to the untreated control group. The clear effect of these treatments can allow for additive or synergistic improvement in combination with candidate molecules.
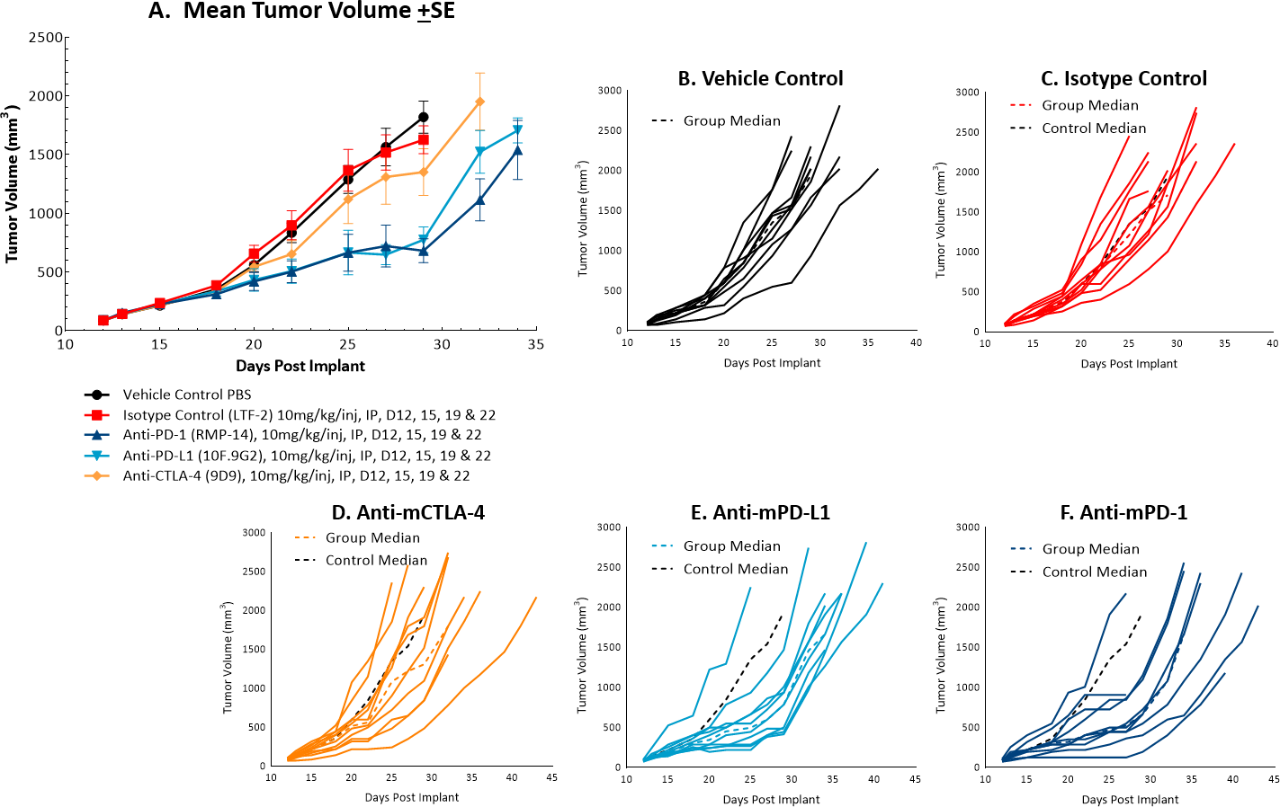
Fig. 1: Mean and individual growth of MC38 tumors following checkpoint inhibitor therapy.
MC38 Tumors Following Costimulatory Antibody Therapy
The response of MC38 to the costimulatory molecules anti-mOX40 and anti-mGITR was also evaluated and we found less robust anti-tumor activity when compared to responses illustrated in Figure 1 (see Figures 2A and 2B-E). Anti-mOX40 treatment produced moderate anti-tumor activity with 51% median ΔT/ΔC at Day 22, 10% putative responders and 4.5 days of tumor growth delay. Treatment with anti-mGITR had the least amount of anti-tumor activity with 72% median ΔT/ΔC at Day 22, 20% putative responders and 2 days of tumor growth delay. However, intimations of activity demonstrating room for improvement are present with these agents as well, making MC38 an attractive model for combination therapy with a wide range of immunomodulatory agents.
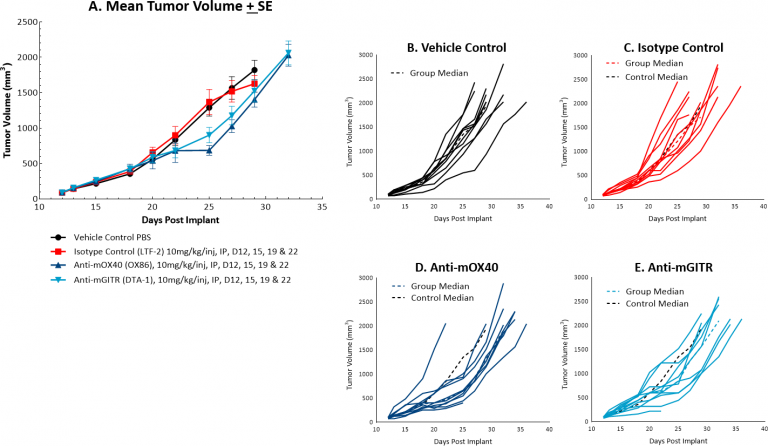
Fig. 2: Mean and individual growth of MC38 tumors following costimulatory antibody therapy.
MC38 Tumors Following Focal Radiation Therapy Alone or in Combination with Anti-mPD-1
While radiation therapy is not commonly utilized in colon cancer, it can be used in certain circumstances such as trying to shrink a tumor prior to surgery or in combination with chemotherapy in patients that may not be healthy enough for surgery. However, in comparison, radiation is used more frequently in rectal cancer. We utilize the Small Animal Radiation Research Platform (SARRP, Xstrahl) to deliver focal beam radiation to murine models. In the subcutaneous MC38 model we tested a single, focal dose of either 5, 10 or 20Gy. We observed a dose response anti-tumor activity following treatment (Figure 3) with 5Gy providing very little activity, 10Gy providing modest activity, and 20Gy providing substantial activity. In follow on work, we tested the combination of 10Gy radiation and anti-mPD-1 in the MC38 tumor model (Figure 4). We found that the combination resulted in improved anti-tumor responses and resulted in 30% tumor free survivors whereas the monotherapies had no tumor free survivors. Therefore, MC38 is also an attractive model to test combination approaches with radiation.
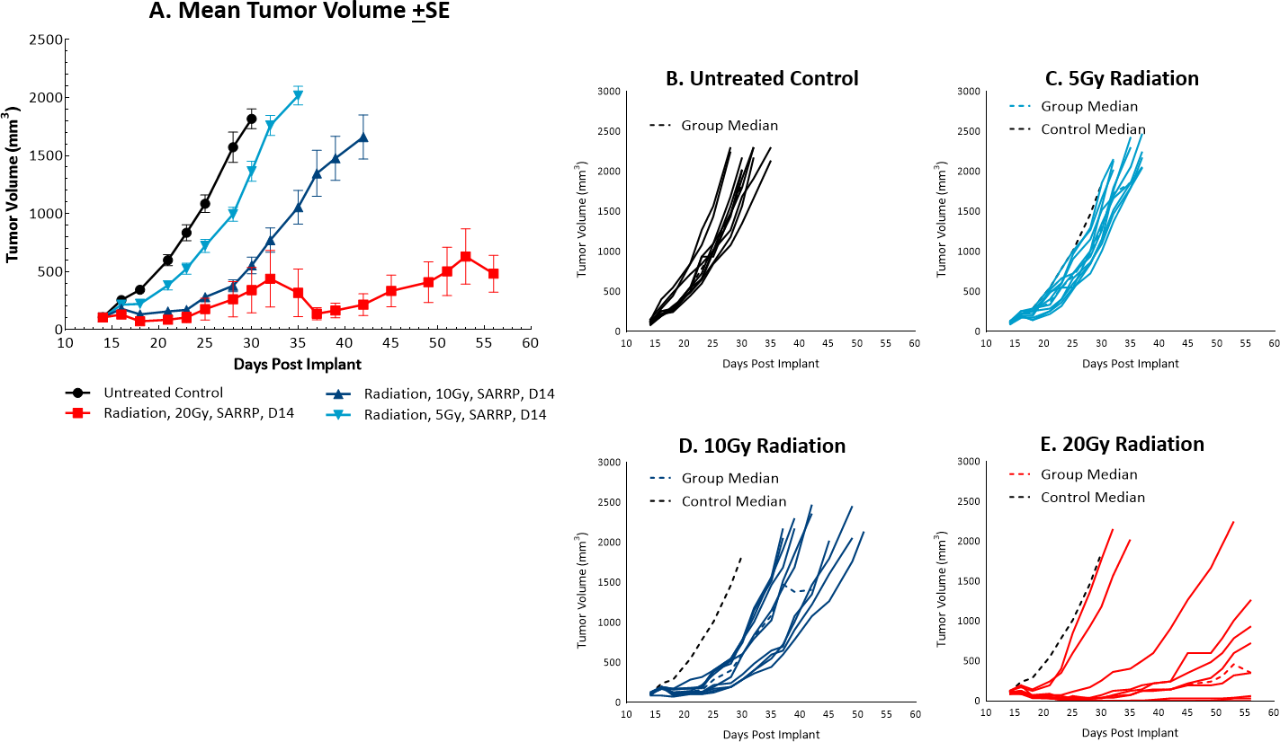
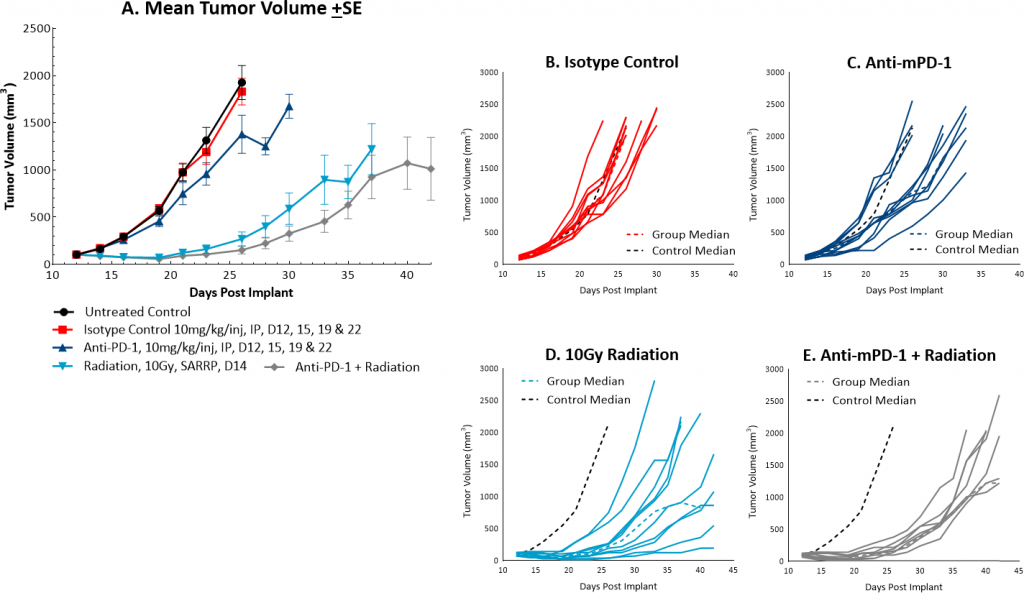
Fig. 4: Mean and individual growth of MC38 tumors following combination treatment with anti-mPD-1 and focal radiation.
The MC38 murine colon carcinoma model can be employed as a robust preclinical immuno-oncology model. Our data supports the use of this tool in investigating novel treatment combinations with radiation, checkpoint inhibitors, costimulatory molecules, or other novel approaches.
Please contact us to speak with our scientists about how MC38 or one of our other syngeneic models can be used for your next immuno-oncology study.
