09 Aug 2019
Author: Erin Trachet | Director, Scientific Development
Date: September 2019
Breast cancer remains one of the most prolific and life-threatening diseases among women worldwide. According to the American Cancer Society, among US women in 2017, there were approximately 253,000 new cases of invasive breast cancer resulting in 41,000 deaths.[1] Approximately 6-10% of new breast cancer cases (15,000-25,000) are diagnosed as metastatic (Stage IV). However, it is thought that 20-30% of all breast cancers will become metastatic over time.[1]
Animal models can be very powerful tools for analyzing the development and progression of cancer. One example of this is the transgenic polyoma middle T oncoprotein (PyMT) model. Expression of PyMT is restricted to the mammary glands by the MMTV promoter/enhancer and results in mice that experience four distinct stages of tumor progression from premalignant to malignant. These stages are comparable to human breast cancer. In addition to the morphological similarities with human breast cancer, the expression of biomarkers in MMTV-PyMT-induced tumors is also consistent with those associated with poor outcome in humans. These include a loss of estrogen and progesterone receptors and the persistent expression of ErbB2/Neu (Her2) and cyclinD1 in MMTV-PyMT tumors as they progress to the malignant stage.[2]
Given the desperate need, and the uniqueness of the MMTV-PyMT model, Labcorp has developed and optimized a transplantable version of this model that’s derived from transgenic host mice (FVB/N-Tg(MMTV-PyVT)634Mul/J).
Primary Mammary Fat Pad Tumor Growth
Orthotopic primary tumor growth kinetics for the transplantable MMTV-PyMT (PyMT) model are shown in Figure 1. The median doubling time is ~8 days with a steady increase in tumor volume and no apparent tumor related body weight loss. As animals were euthanized due to primary tumor growth, lung nodules were noted. The growth rate allows for a four-week therapeutic window to evaluate anti-tumor responses.
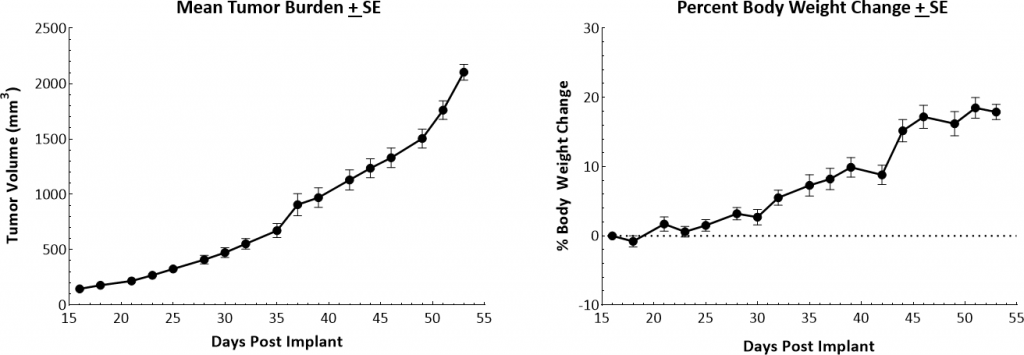
Fig. 1: Growth kinetics and body weight changes of MMTV-PyMT tumors in female FVB/NJ mice.
Immune Profile of PyMT Tumors
The baseline tumor immune composition was analyzed by flow cytometry and is shown in Figure 2. The lymphoid population has an equal distribution of CD8+ and CD4+ T cells. While NK cells have a considerable presence, B cells and NKT cells are minimally represented. The tumors have a large myeloid population represented predominantly by G-MDSCs and M1-TAMs. The infiltration of CD8+ T cells and NK cells supports the theory that the PyMT model could be responsive to immune modulatory agents. However, there are also considerable myeloid suppressive cells within the tumor microenvironment. Thus, we tested response to immunomodulatory agents (see Figure 3), to determine if the PyMT model is more in line with an immunosuppressive “cold” tumor or an immune-responsive “warm” tumor.
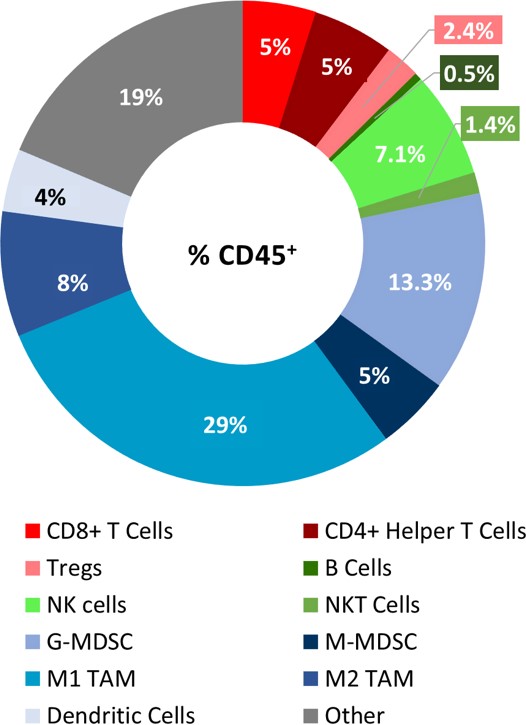
Fig 2: Immune profile of PyMT tumor infiltrates
Response to Checkpoint Blockade and Focal Radiation Treatments
To evaluate the anti-tumor response to immunomodulatory agents, mice bearing orthotopic PyMT tumors were treated with checkpoint blockade antibodies (anti-mPD-1, anti-mPD-L1, and anti-mCTLA-4) and focal radiation. Treatment with single agent checkpoint inhibitors showed a mild response resulting in tumor growth delays ranging from 3-6 days but no tumor regressions (Figure 3). Orthotopic PyMT tumors are only marginally sensitive to treatment with checkpoint inhibitors, leaving a clear opportunity for combination therapy to show added benefit. In addition, this data suggests that the model fits into more of a “cold” tumor profile.
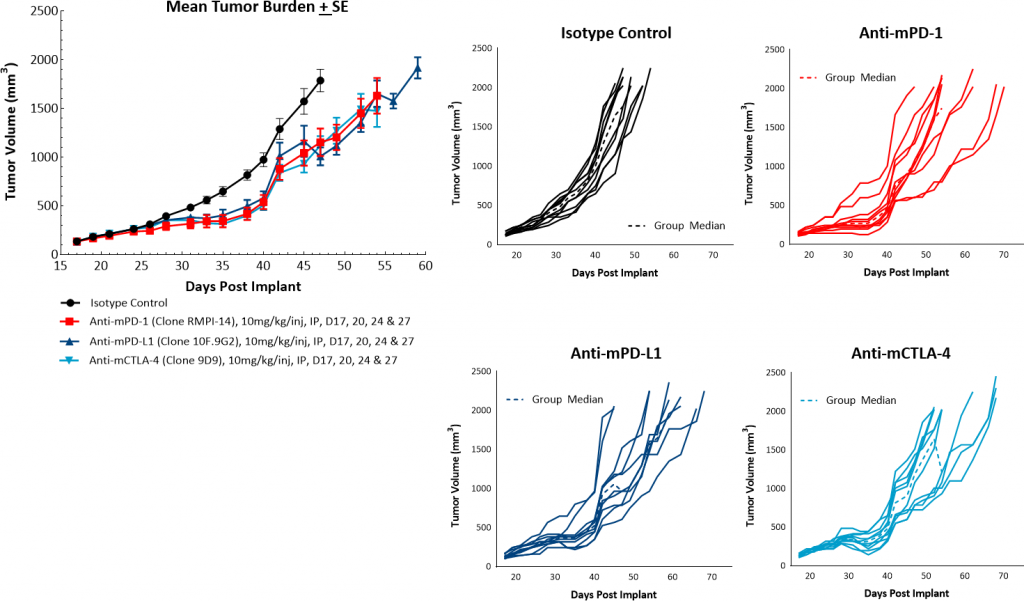
Fig. 3: PyMT response to checkpoint inhibitors.
In addition to checkpoint blockade, focal beam radiation is also thought to be a useful treatment approach to modify an immunosuppressive tumor microenvironment. Furthermore, radiation treatment is a common therapy for many breast cancer patients. Labcorp utilizes the Small Animal Radiation Research Platform (SARRP; Xstrahl) to provide focal radiation therapy to mice in a manner consistent with radiation treatment in patients. In this model we tested single, focal radiation doses of 5, 10, or 20Gy and found them to be well tolerated and highly effective. Both the 20 and 10Gy dosage levels produced strong anti-tumor responses with 100% and 38% incidence of complete responders, respectively (Figure 4). Based on this data, a single dose of 5Gy focal radiation would be recommended for combination studies.
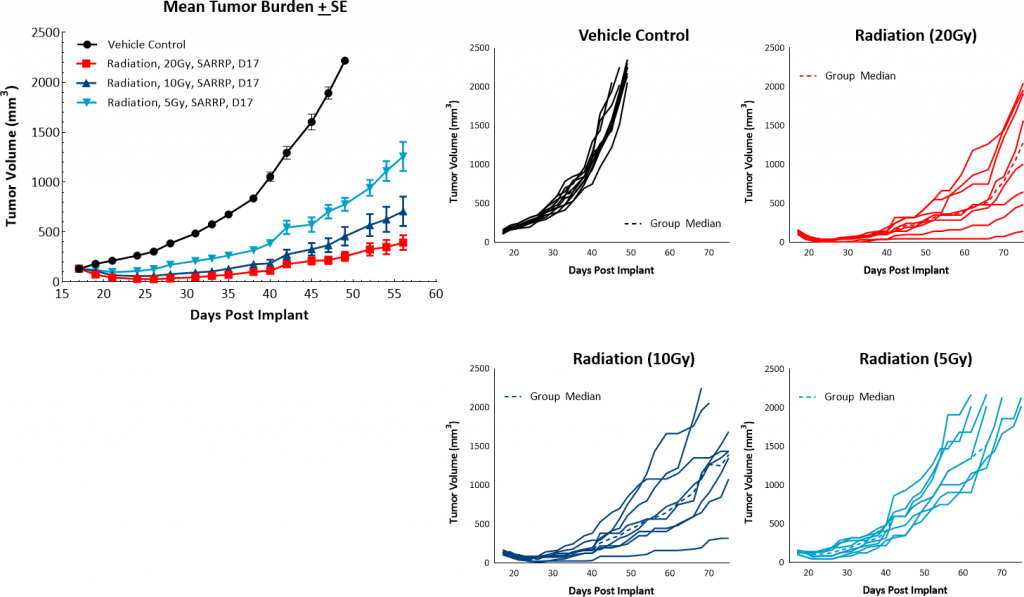
Fig. 4: PyMT response to focal radiation.
Overview
Transplantable PyMT is a very useful model to evaluate drugs targeting breast carcinoma. This model offers the research community the ability to utilize a model that mimics many aspects and complexities of the clinical disease. Many clinical approaches in breast cancer are now incorporating immunotherapies and/or radiation therapy as part of ongoing clinical trials. The data we outline here suggests that the PyMT model is appropriate for combination studies with either checkpoint inhibitors or low dose focal beam radiation. Contact Labcorp to learn more about our transplantable PyMT model or other breast cancer models that we offer.
