01 Jun 2017
Author: Maryland Franklin, PhD, Vice President, Scientific Development
Date: June 2017
While the direct contribution of CD19 to human B cell cancers is still under investigation, its expression is found on a majority of B cell malignancies. For example, acute lymphoblastic leukemia (ALL), B cell lymphomas, and B cell leukemias demonstrate 80%, 88%, and 100% CD19 expression, respectively.1 ALL presents with an overproduction of lymphoblasts in the bone marrow that continuously multiply resulting in the inhibition of normal hematopoietic cell production.
ALL is more commonly found in young children but also arises in older adults. Children have a >80% cure rate while adults only have a 20-30% cure rate. A similar disease is chronic lymphocytic leukemia (CLL) which is generally diagnosed in older adults. Both ALL and CLL have been identified as malignancies suitable for CD19-directed therapies. While anti-CD19 monoclonal antibodies and other approaches are being investigated for clinical development, by far the most common approach for targeting CD19-related malignancies are the Chimeric Antigen Receptor (CAR) modified T cells (CAR-T). CD19 was the first target used for this T cell therapy strategy and still remains an area of active clinical investigation.
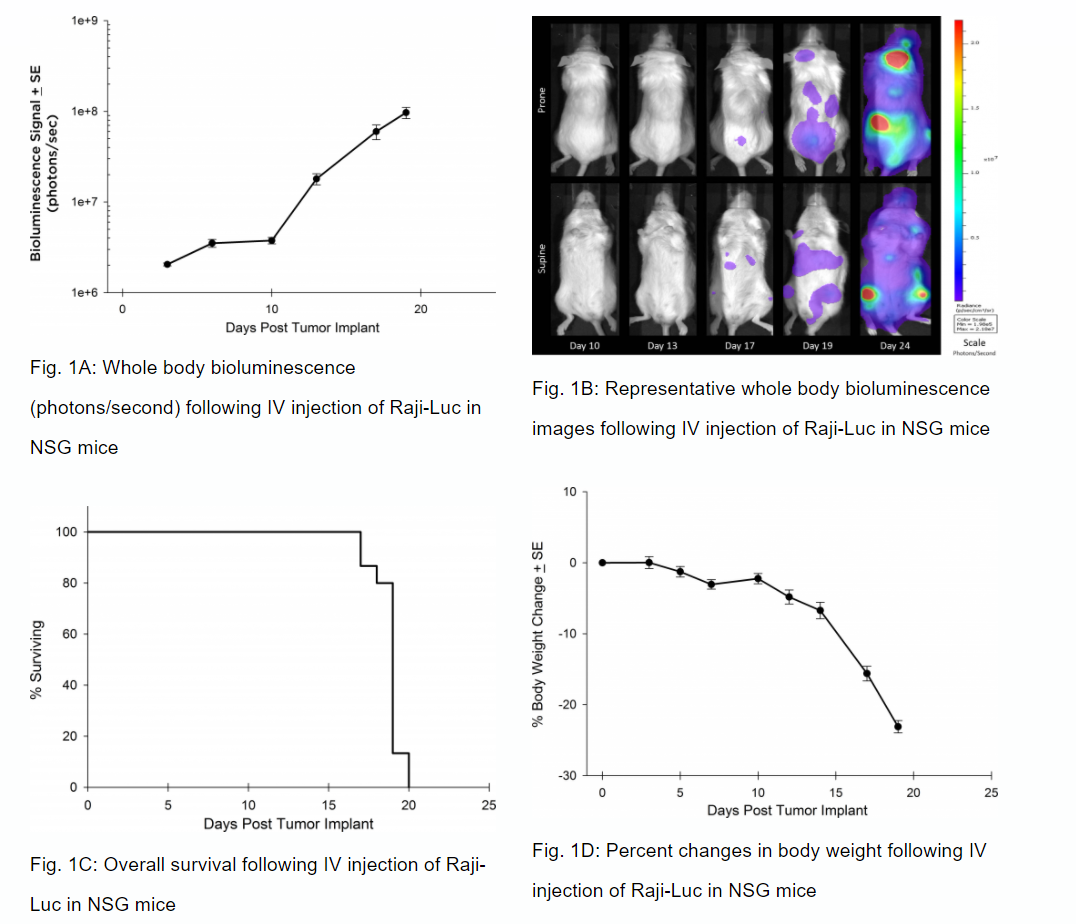
Preclinically, the human Burkitt’s lymphoma cell line Raji has been a mainstay for determining anti-tumor activity for approaches related to B cell malignancies. At Labcorp, we employ a luciferase-expressing Raji cell line (Raji-Luc) to allow us to track disease progression and therapeutic response over time through in vivo optical imaging. Raji-Luc is an excellent model to evaluate CD19-directed CAR-T therapies. We have utilized this model in both SCID and NSG mice for more than a dozen CAR-T or other cellular therapy studies. In many instances the most immunodeficient mouse strain, NSG, is required for survival and persistence of the cellular therapy. Thus, we validated growth of Raji-Luc in NSG mice by in vivo bioluminescence imaging (BLI) (figures 1A & B) and found an aggressive dissemination of disease as illustrated by an approximately 20 day overall survival (morbidity/mortality, figure 1C) endpoint. Consistent with the rapid induction of disease, the mice presented with progressive body weight loss (figure 1D) and ultimate hind-limb paralysis that required euthanasia.
Validation of Raji-Luc Growth in NSG Mice
Anti-tumor Activity of Anti-CD20 Monoclonal Antibody—Rituximab
Similar to CD19, CD20 is also a marker of B cells and was an initial target of drugs focusing on B cell malignancies like Non-Hodgkin’s lymphoma. The clinical development of the anti-CD20 monoclonal antibody, rituximab, proved ground-breaking for use of monoclonal antibodies as oncology therapeutics. In SCID mice, we have tested the anti-tumor activity of rituximab and demonstrated increased overall survival (figure 2A) that correlated with reduced whole body tumor burden determined by in vivo BLI (figures 2B and 2C). Similar to studies in NSG mice, we observed clinical signs of disease progression like hind-limb paralysis and body weight loss (figure 2D). In addition to in vivo BLI we also examined incidence of disease in various tissues with ex vivo BLI (table 1). Through ex vivo anlayses, we further demonstrated that treatment with rituximab reduced overall disease burden.
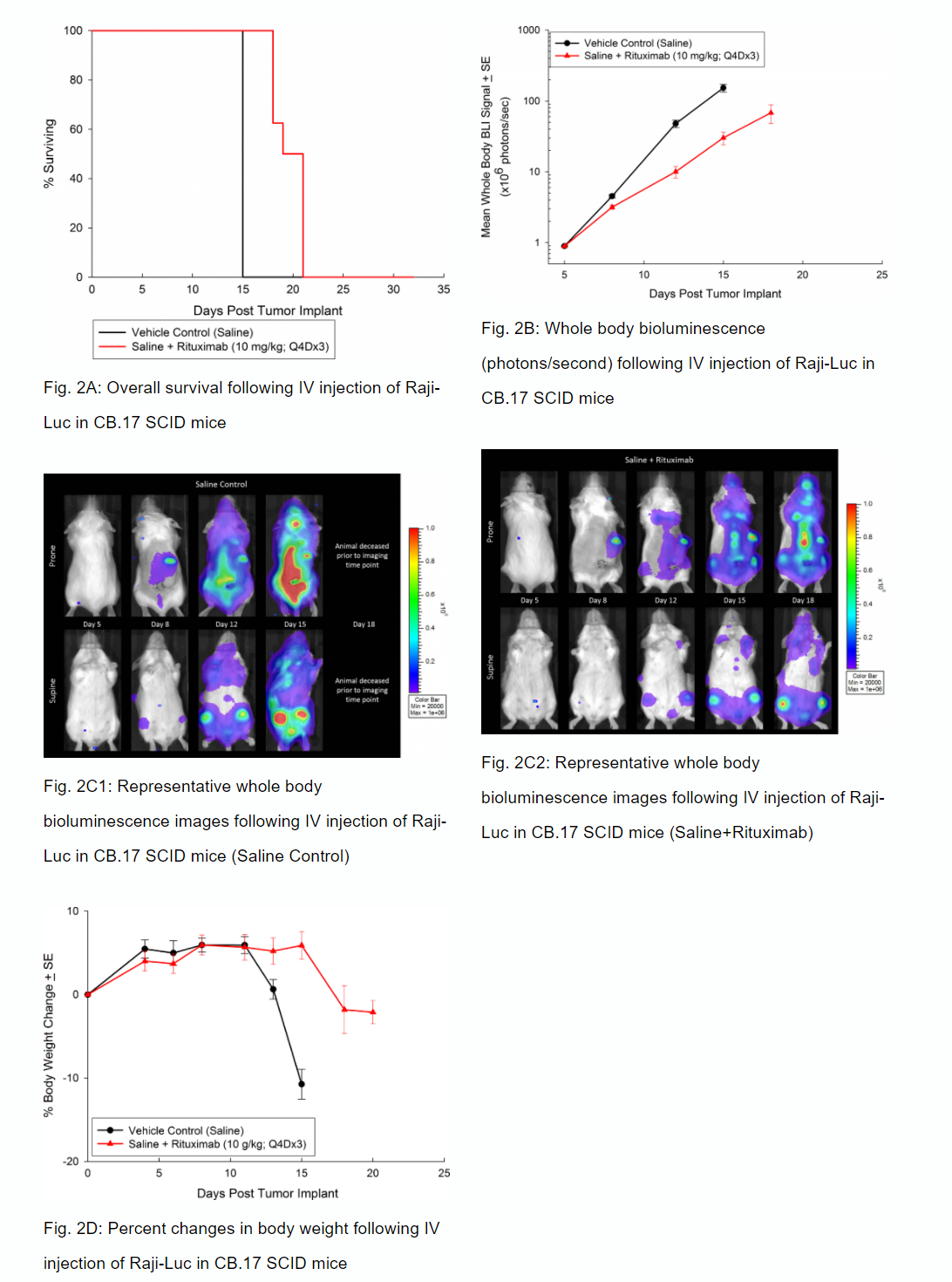
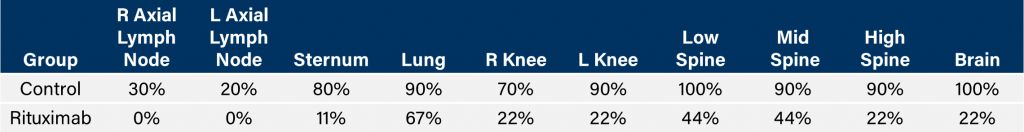
Table 1: Incidence of disseminated disease following IV injection of Raji-Luc in CB.17 SCID mice.
Evaluation of Raji-Luc Model in SC Setting in SCID Mice
While most therapeutic approaches have utilized the Raji-Luc model following IV implantation, there have been some instances where anti-tumor strategies need subcutaneous (SC) evaluation. To this end, we validated growth of the Raji-Luc line in the SC setting in SCID mice. The line grows quite rapidly with a four-day doubling time and about 27 days from implant until euthanasia criteria are met (figure 3).
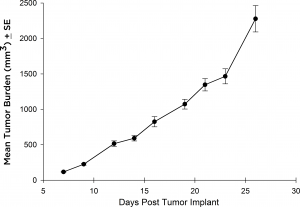
Fig. 3: Subcutaneous growth of SC implanted Raji-Luc cells in CB.17 SCID mice
Thus, Raji-Luc in either the SCID or the NSG background provides a rapid model with which to test CD19 or CD20 directed therapies.
Contact us to speak with one of our scientists about how Raji-Luc or one of our other models can be used for your next CAR-T study.
