It is estimated that in 2017 there will be about 30,000 new cases diagnosed and about 13,000 patient deaths from multiple myeloma. However, it is a relatively uncommon type of cancer with a lifetime risk of 1 in 143 (0.7%). The risk of being diagnosed with multiple myeloma increases as people age, with the majority of people being diagnosed at age 65 or older. Men are slightly more likely to develop multiple myeloma than women and African Americans are twice as likely to develop this type of cancer. However, the greatest risk factor for begin diagnosed with multiple myeloma is already having a plasma cell disease. Patients with monoclonal gammopathy of undetermined significance (MGUS) or solitary plasmacytoma will eventually develop multiple myeloma.
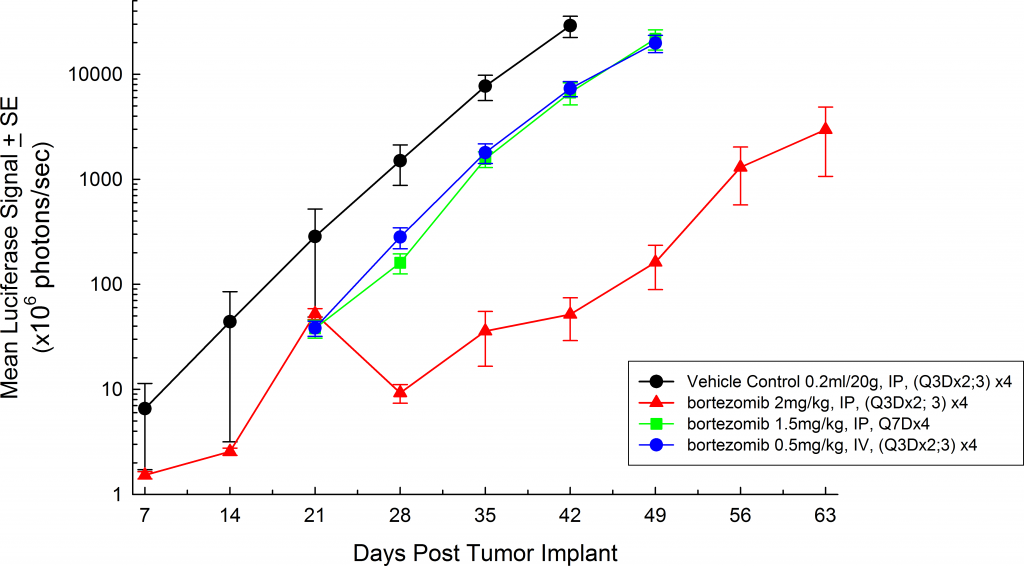
Multiple myeloma is a clonal B cell malignancy characterized by the accumulation of terminally differentiated, antibody-producing plasma cells in the bone marrow. Genetic mutations within the myeloma cells and their interaction with various cytokines and growth factors contribute to the invasiveness of the disease and enhanced drug resistance. Patients are generally asymptomatic until very late state disease. Once disease is detected, localization in the bones, especially the spine, is common. Currently, the gold standard therapy is a combination of doxorubicin and dexamethasone with bortezomib. The addition of bortezomib improves the overall response rate close to 50%.1
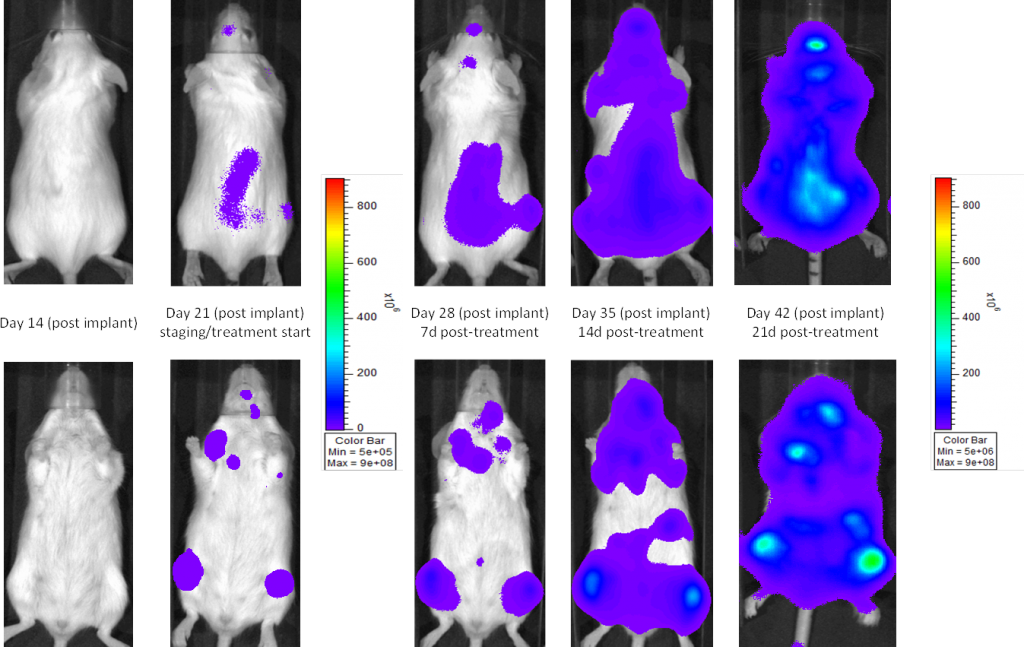
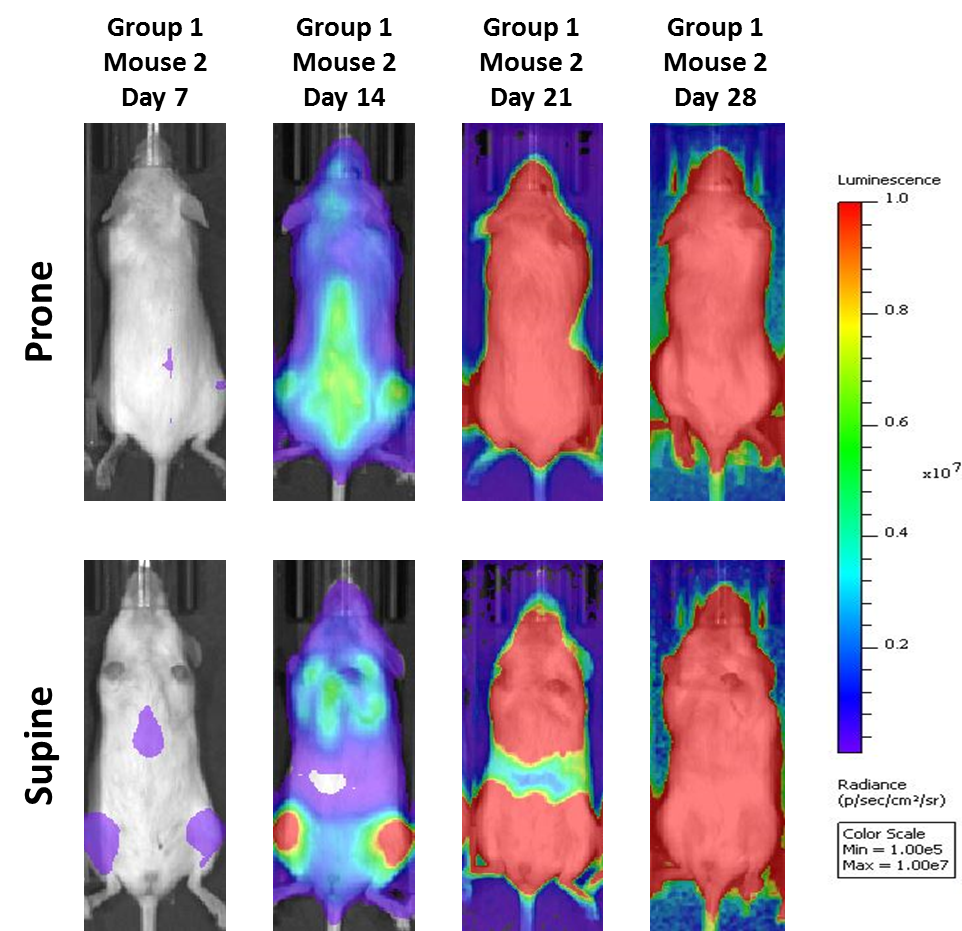
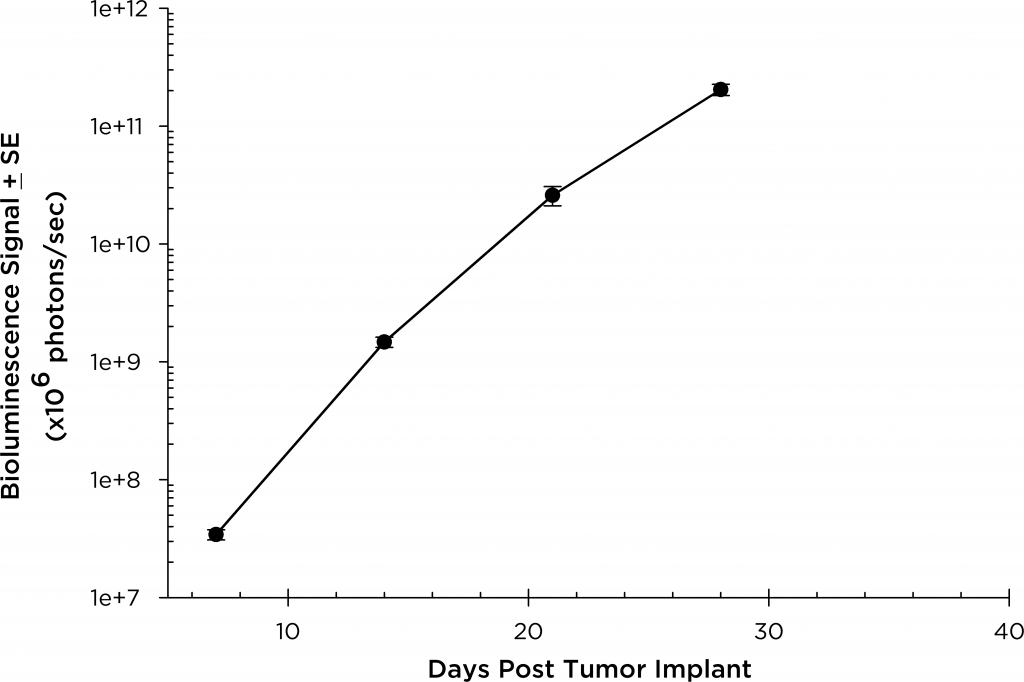
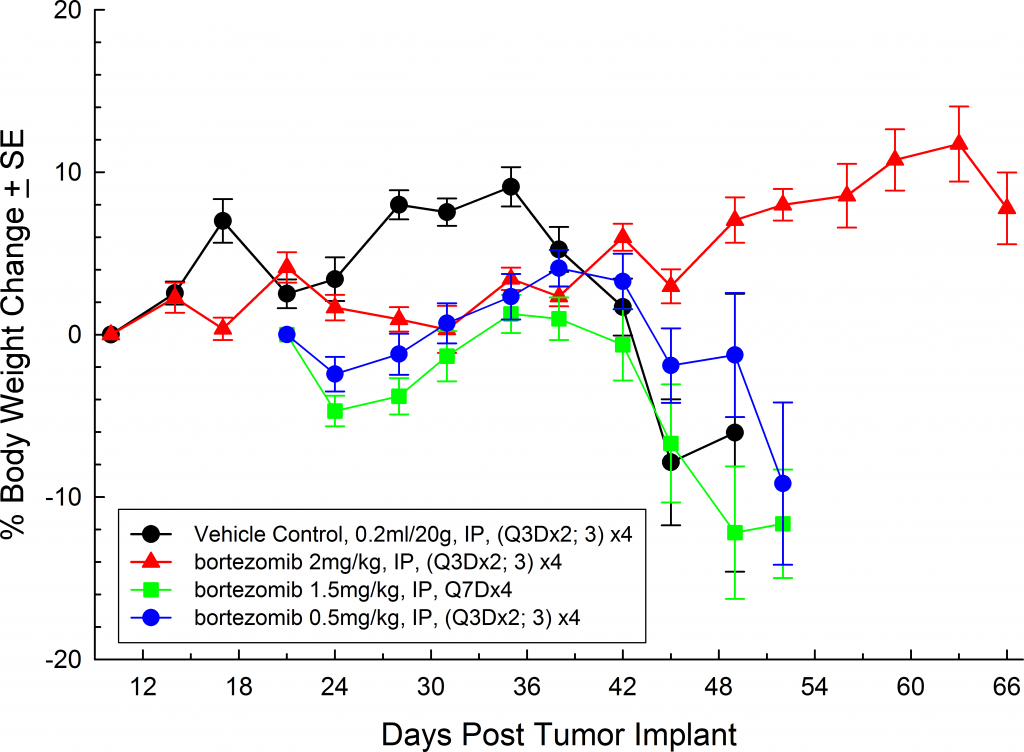
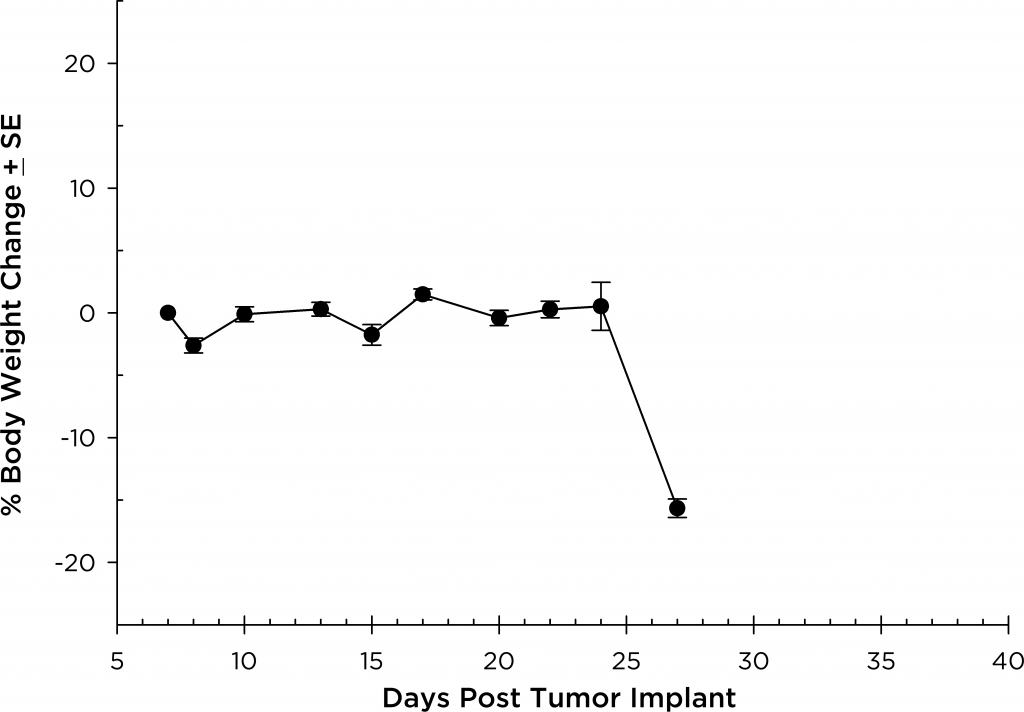
A commonly used cell line to evaluate novel therapies for multiple myeloma is MM.1S. MM.1S was derived from a 42 year old African American woman and has been documented to express CD25, CD38, CD52, and CD59. It also expresses the glucocorticoid receptor and is dexamethasone sensitive.2 In an effort to more effectively monitor in vivo disease progression, we transfected the MM.1S line with luciferase (MM.1S-pMMP-LucNeo). With bioluminescence imaging (BLI) we can monitor disseminated disease progression over time and find reproducible in vivo growth in mice (Figures 1 and 2).