As we presented in last month’s model spotlight, lung cancer is a devastating disease and is the leading cause of cancer death in the US and worldwide.1 The research community continues to look for new models that will aid in lung cancer research. The ATCC (a widely used cell repository) currently has over 100 different human derived lung cancer cell lines.
Because lung cancer is so prevalent, there are numerous opportunities for new human lines to be acquired and characterized. Scientists have become highly skilled in distinguishing the mutations that drive increased proliferation from those that suppress tumor growth. This information is key to developing novel therapies to treat lung cancer.
Labcorp continues to investigate new and/or unconventional cell lines and understand their importance to our client’s research. In this Model Spotlight we will discuss a few of these lines and how we position our expertise to be a valuable resource to our clients as they look to develop future treatments.
NCI-H3122
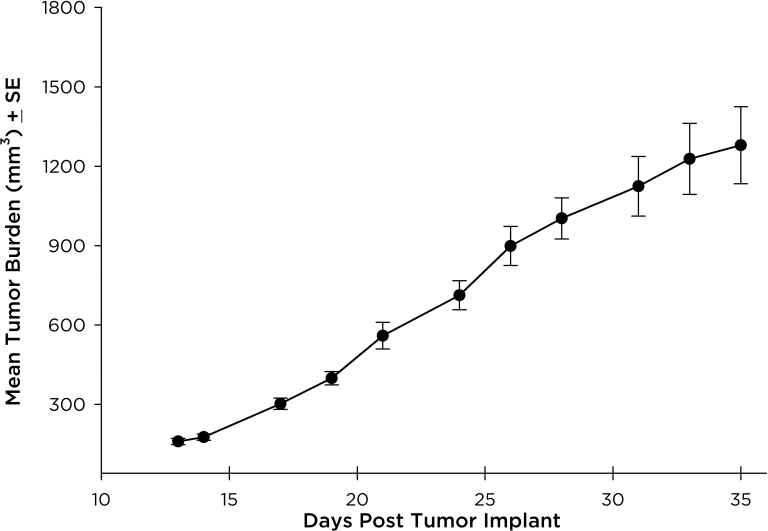
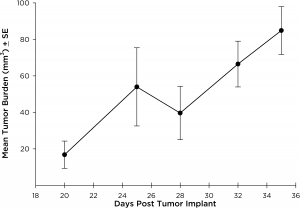
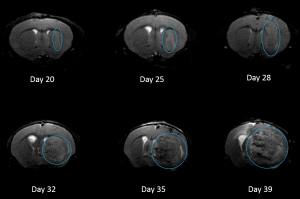
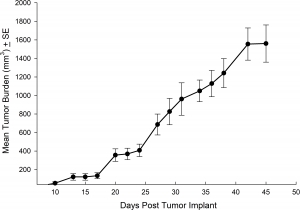
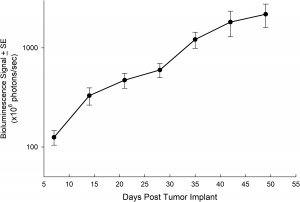
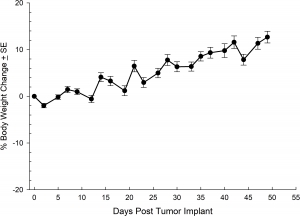
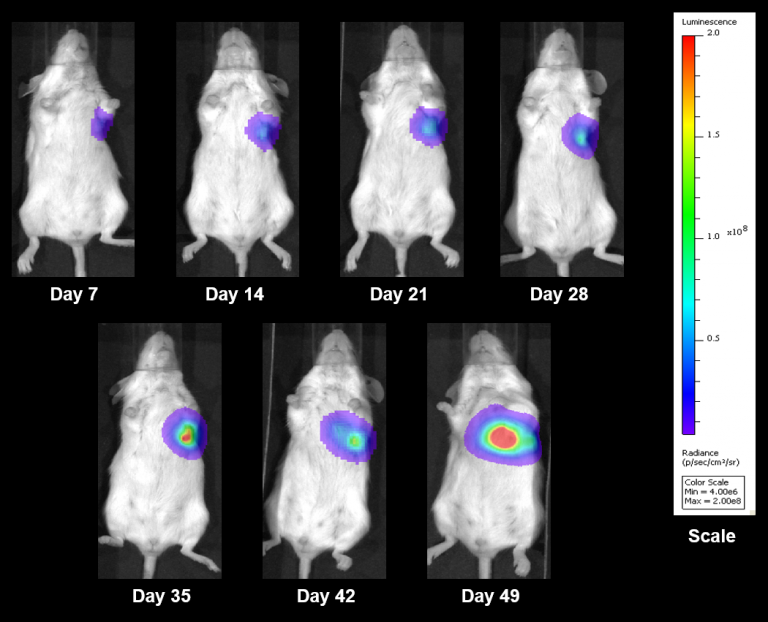
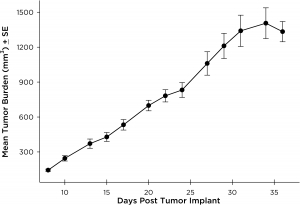
NCI-H3122 was derived in 1981 from a primary bronchioalveolar carcinoma of the lung from a 52-year-old male taken prior to treatment. This cell line harbors the EML4-ALK fusion protein (echinoderm microtubule-associated protein-like 4 fused with anaplastic lymphoma kinase gene) and has recently been identified in ~7% of Japanese NSCLC (non-small cell lung cancer) patients and ~4.5% of all NSCLC cases. The ALK gene is a target of interest given its ability to be oncogenic. As NCI-H3122 contains EML4-ALK and is highly sensitive to single agent ALK inhibitors it appears that its survival is mediated by a more ALK-addicted pathway.2 As a preclinical model this cell line would be suitable for screening novel ALK inhibitors that are not dependent on EGFR, or other pathways involved in NSCLC. The model has been developed as a subcutaneous (SC) implant; however, we have also implanted this line intracranially to mimic the clinical setting where advanced NSCLC metastasizes to the brain. Subcutaneous tumor growth is reliable with minimal animal-to-animal variability. The tumor volume doubling time in the SC setting is ~7 days and mice typically reach an evaluation size of ~750mm3 in about 25 days. Initial data with NCI-H3122 implanted intracranially is encouraging, in a pilot study we have seen 100% take rate and a tumor volume doubling time, based on volumetric assessment, of ~7 days, which is consistent with the SC growth rate (See Figures 1 and 2). As with many intracranial models, morbidity (body weight loss and overall clinical observation) is used to define the overall survival. For this cell line the median day of death is ~35 days (See Figures 2, 3, and 4).