Pancreatic cancer is the ninth most commonly diagnosed cancer and ranks as one of the deadliest with the lowest 5-year survival rate of 5-8%.[1,2,3] This year alone, the American Cancer Society estimates that 56,770 people will be diagnosed with the disease of which more than 45,750 people may not survive. Pancreatic ductal adenocarcinoma (PDAC) being the most prevalent and aggressive form of exocrine pancreatic cancer accounts for ~90% of cases with mortality equaling incidence.[4,5]
At diagnosis, a vast majority of PDAC patients have either locally advanced, unresectable, or metastatic disease. The silent spread of the disease, and appearance of disease specific symptoms only at advanced stages, makes early detection very challenging and contributes to the low survival rates. Treatment options for resectable patients include surgery followed by first line systemic chemotherapy (gemcitabine, FOLFIRINOX, paclitaxel) or combination chemoradiotherapy. For locally advanced, unresectable patients, the regimen includes multi-agent chemotherapy (5-FU, oxaliplatin, irinotecan) or chemoradiotherapy.[6] As these treatments have offered limited benefits to pancreatic cancer patients, newer options like immunotherapy drugs are now being tested in clinical trials.
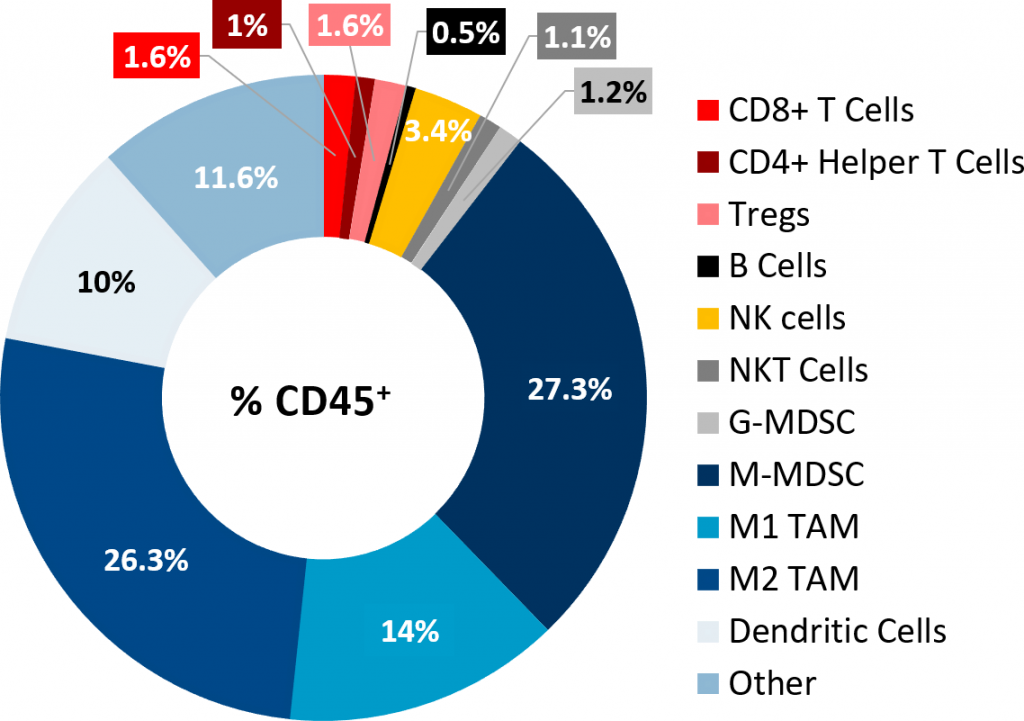
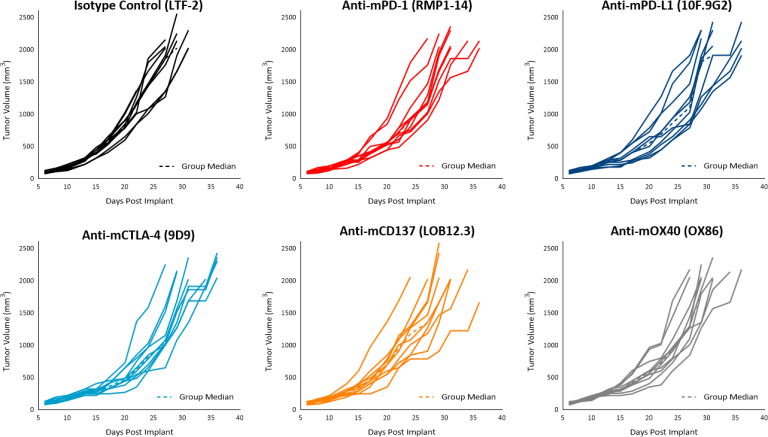
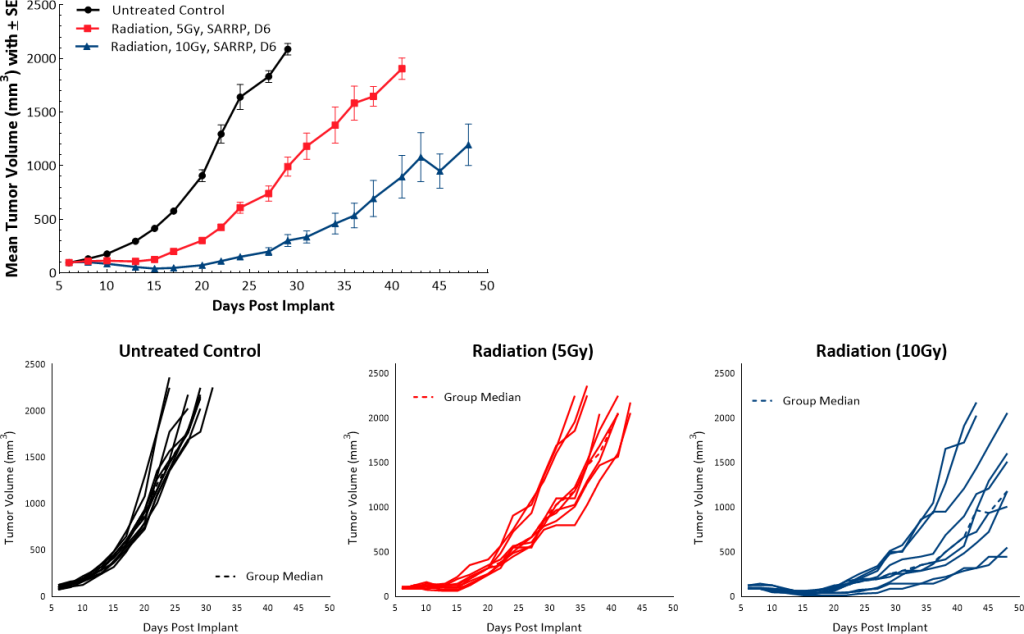
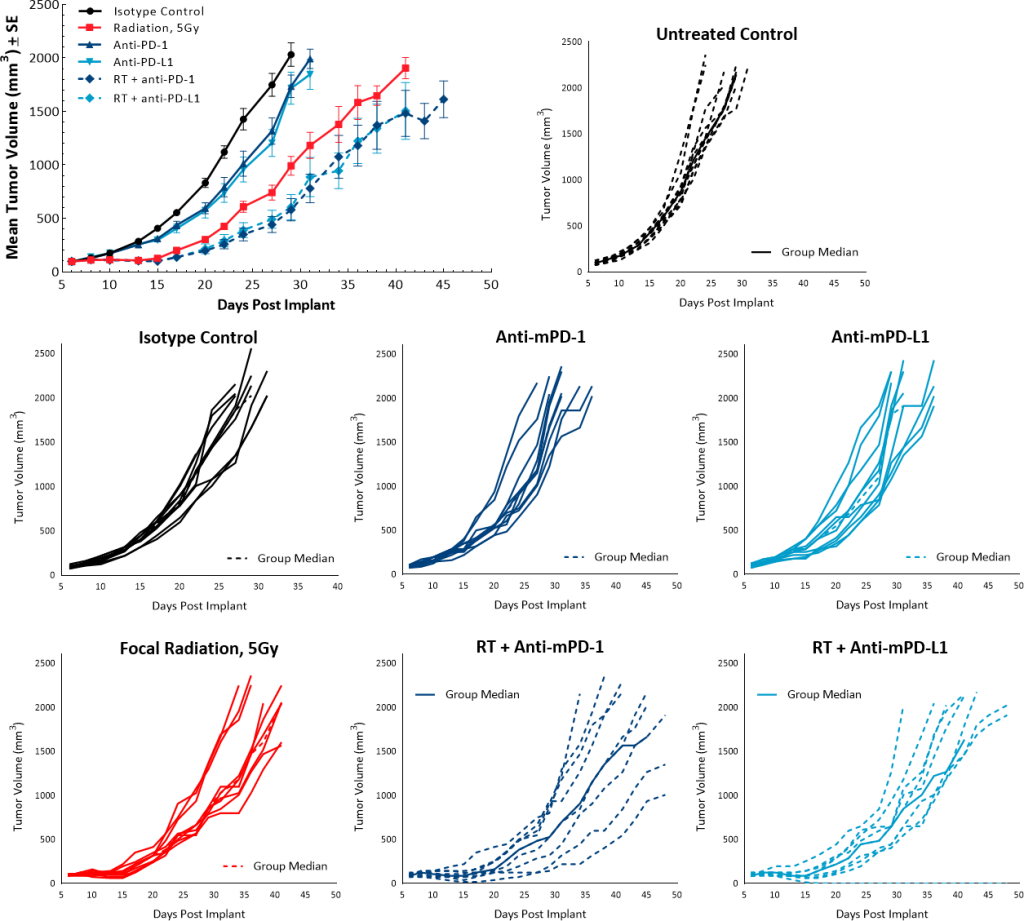
PDAC are characterized by presence of fibrotic stroma, low mutational burden, lack of CD8+ T cell infiltration and presence of immunosuppressive myeloid cell populations, all of which contribute to the typical non-immunogenic tumor characteristics.[7] Current approaches to enhance treatment response focus on increasing trafficking of T cells to the tumors using agents like radiotherapy (RT). RT alone, in most solid tumors, has the potential to activate the immune system to trigger an anti-tumor immune response following cytotoxic death and release of immune stimulating signals. However, in PDAC patients, the outcome of high dose RT alone, or in combination with chemotherapy agents, remains generally unsatisfactory[8] and clinical trials with single agent immunotherapy agents indicate poor response rates as low as 1-2%.[9] At least 21 open clinical trials are currently investigating the combination of RT with other immune-stimulating agents which may be a promising avenue for PDAC treatment and merits further research.[10] Along the same line, in the preclinical platform we are evaluating single and combination therapies using the Pan02 murine PDAC model. Pan02 is a well-established Grade III adenocarcinoma model developed by chemical induction with 3-MCA (3-methylcholanthrene) in male C57BL/6 mice.[11] In this model spotlight we present in vivo data on Pan02 tumor model growth kinetics, response to immunomodulatory antibodies, focal radiation, and the combination of RT with immune modulatory agents tested in the syngeneic C57BL/6 mouse strain.
Pan02 Growth Parameters
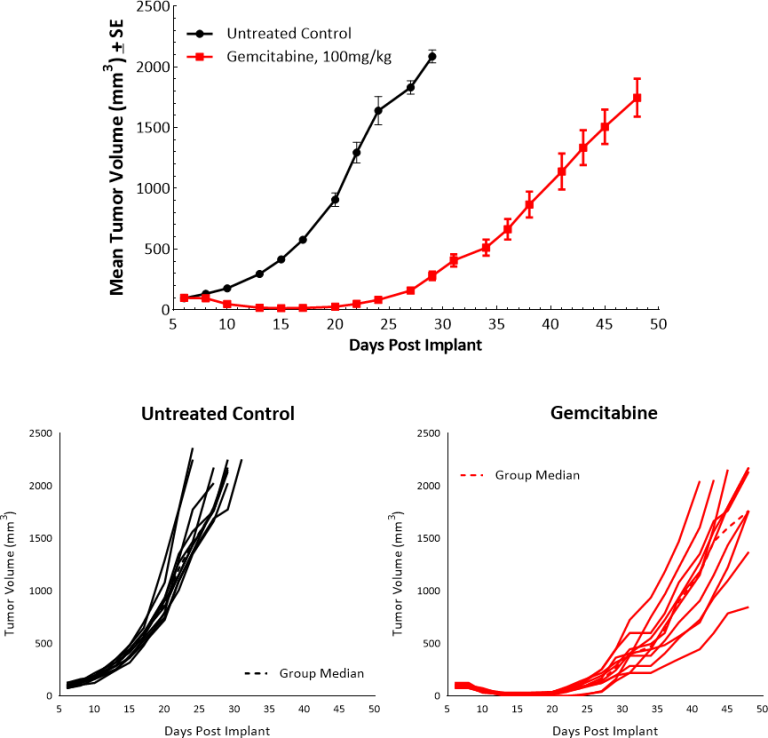
Subcutaneous implant of Pan02 results in successful establishment of tumors that have a median doubling time of 4-5 days and mice stay on study for ~30 days post implant (Fig. 1) with no weight loss (data not shown). The growth kinetics allow for over a three-week therapeutic window to evaluate anti-tumor responses in this model.