Kidney cancer generally occurs in older people, with an average age of diagnosis at 64 years old. Kidney cancer is among the ten most common types of cancer in the United States, with an overall lifetime risk of approximately 1.6 percent. The most common form of kidney cancer is renal cell carcinoma (RCC), representing 90-95 percent of all cases. Unfortunately, patients are asymptomatic until advanced stage of the disease, leaving them with limited treatment options. Given the poor prognosis, which is an eight percent 5-year survival rate for patients with advanced (stage IV) disease, predictive preclinical models are needed for reliably evaluating treatment options. While subcutaneous xenograft models are valuable, they fail to capture the complexity of the cancer growing in the tissue of origin. Orthotopic models provide an avenue for not only evaluating the treatment response, but also the impact on the origin tissue and tissue microenvironment-which can be critically important in trying to preserve kidney function.
786-O (pMMP-LucNeo) – A model for renal cell carcinoma
Date: September 2016
Author: Erin Trachet, Sr. Scientific Advisor, Oncology / Sr. Manager, Proposal Development
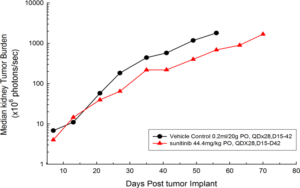
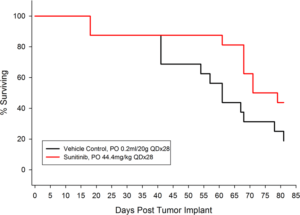
Figure 1: Median BLI Signal for Orthotopic 786-O (pMMP-LucNeo) Human Renal Carcinoma
Figure 2: Percent Survival for Orthotopic 786-O (pMMP-LucNeo) Human Renal Carcinoma
In an effort to address this unmet need, we have developed an orthotopic RCC model, 786-O (pMMP-LucNeo) through implantation into the sub-renal capsule. This cell line has been transfected with luciferase, which allows for disease and response monitoring through bioluminescence imaging (BLI). In 786-O tumor bearing mice, we evaluated the anti-cancer activity of sunitinib. While treatment failed to produce clinically relevant activity, there was a measurable difference between the control and the sunitinib treated groups. Over time, we have found that the tumor growth of this model is reproducible and reliable, making it useful for testing novel therapeutics targeting RCC.
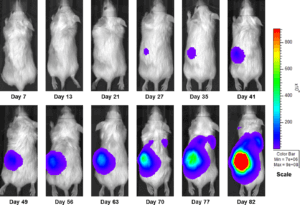
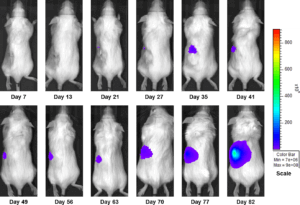
Figure 3a: Representative Images of Disease Progression – Vehicle Control
Figure 3b: Representative Images of Disease Progression – Sunitinib 60mg/kg PO, QDX28
References
Note: Please note that all animal care and use was conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.


