Poster
EMT6-Luc: In vivo monitoring of metastatic development and treatment efficacy
The EMT6 model was derived from a transplanted hyperplastic alveolar metastatic lung nodule in BALB/c mice.4 As further data was generated with this model, observations of metastatic foci in the lung visible upon gross necropsy occurred in up to 40% of control mice. As metastasis is common in breast cancer patients, it was important to take advantage of this finding, so a luciferase enabled EMT6 cell line (EMT6-Luc) was generated to monitor thoracic metastasis by bioluminescence imaging (BLI) in vivo. For this project, animal care and use was conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.
In this spotlight, we present data from our initial efficacy study with the EMT6-Luc model implanted into the mammary fat pad (MFP), both by caliper measurement of the primary tumor and by BLI of the thoracic area to monitor metastatic disease progression. Control tumor growth for the EMT6 and EMT6-Luc models are shown in Figure 1. For both parental and EMT6-Luc models, the median doubling time of MFP tumors is 5.5 days, allowing for a therapeutic window of two to three weeks with which to evaluate anti-tumor activity of test agents.
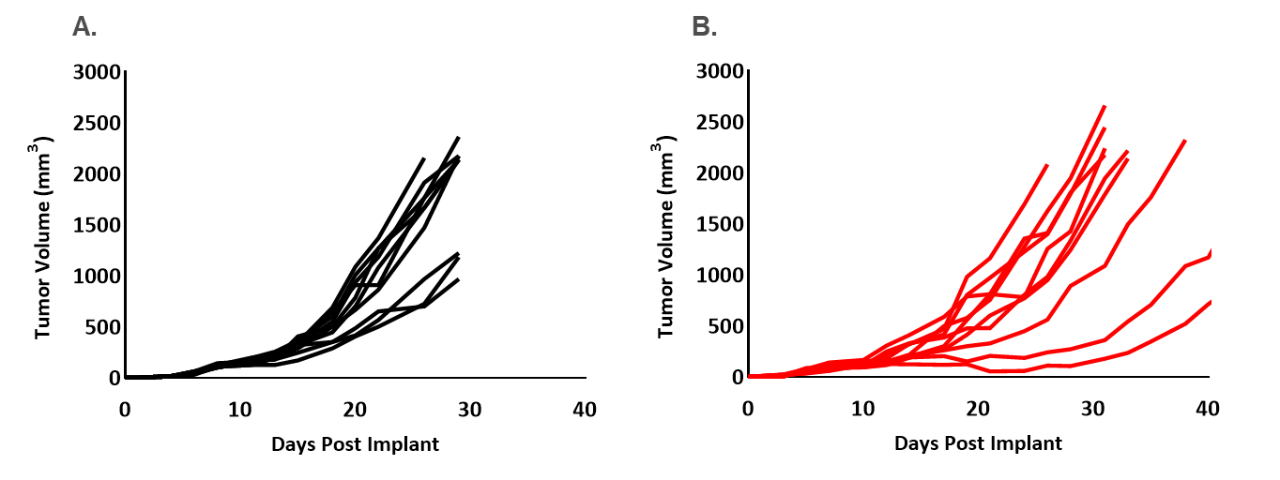
Figure 1. Individual control growth of EMT6 (A) and EMT6-Luc (B) implanted orthotopically (mammary fat pad) into female Balb/c mice.
To determine the response of EMT6-Luc to checkpoint blockade, mice bearing EMT6-Luc tumors were treated with anti-mPD-1 or anti-mPD-L1. Responses to checkpoint blockade were moderate compared to control (Figure 2) and responses to anti-mPD-1 in EMT6-Luc were similar to those data generated with the parental line (Table 1). Response to anti-mPD-L1 seems slightly improved in the EMT6-Luc compared to the parental line (Table 1), but the overall impact of the addition of the luciferase tag with regard to anti-tumor efficacy under the conditions tested appear minimal.
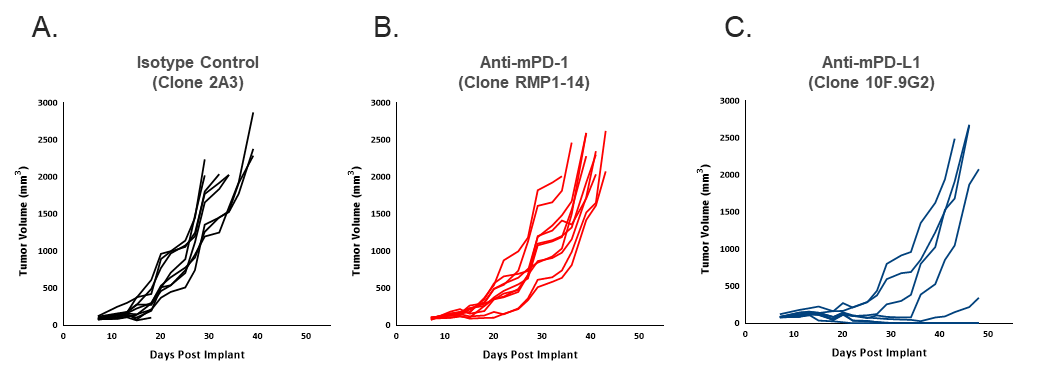
Figure 2. Individual EMT6-Luc tumor volume measurements showing anti-tumor efficacy of anti-mPD-1 (B) and anti-mPD-L1 (C) compared to isotype control (A). Each antibody was dosed twice weekly at 10 mg/kg.
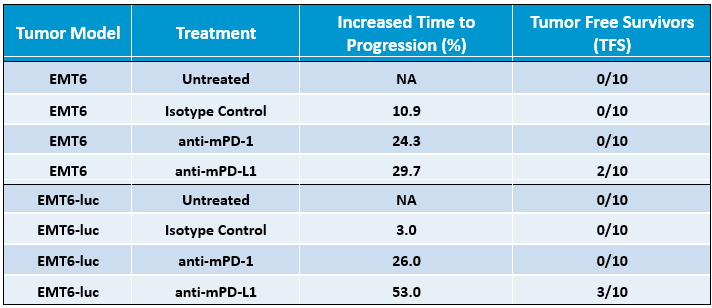
Table 1. Differential responses of EMT6 and EMT6-Luc tumor models with response to checkpoint inhibition by anti-mPD-1 and anti-mPD-L1. Increased Time to Progression endpoint is based on the increase in number of days, compared to untreated tumors, between initiation of treatment and the day of animal exit from the study.
In addition to evaluating response to treatment by primary MFP tumor measures, the luciferase enabled cell line also allows for monitoring the development of thoracic metastasis by assessing BLI signal localization in vivo. The development of thoracic signal is illustrated in Figure 3. As expected, monitoring by BLI offers additional granularity on the extent to which metastasis occurs, as well as any delay in metastatic involvement with treatment of anti-mPD-1 or anti-mPD-L1. All control animals exhibited increasing thoracic BLI signal during the study, but none of these animals exhibited metastatic nodules upon gross necropsy (data not shown). While pathological evaluation is necessary to confirm the etiology of increasing thoracic signal, historical observations of lung nodules upon necropsy in untreated animals strongly suggest that the increasing signal is due to metastases, highlighting the value of this endpoint. Taking this assumption, one can conclude that treatment with anti-mPD-L1 resulted in delayed onset of metastasis in agreement with the delay in primary tumor growth. It should be noted that metastasis in this model does not cause a decline in health, as mice exit the study for primary tumor burden prior to health impact from metastatic involvement.
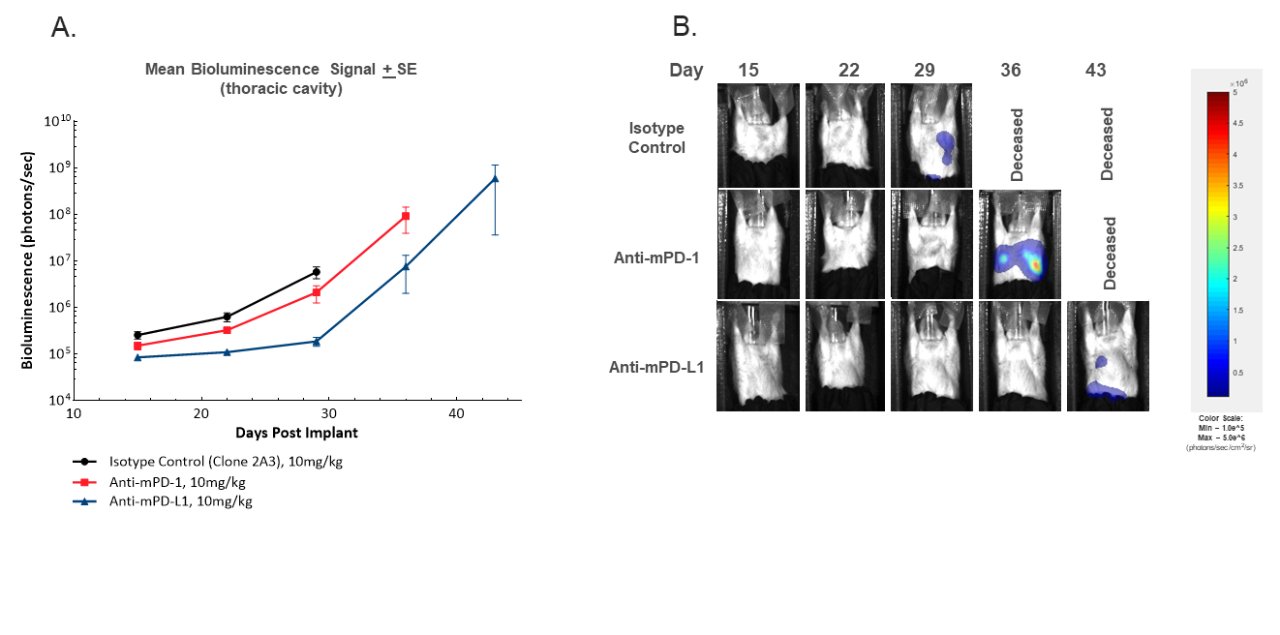
Figure 3. In vivo BLI imaging to monitor development of thoracic signal over time by mean tumor burden in the thoracic region (A) or representative BLI images (B).
When interrogating models for immuno-oncology purposes, it?s also important to know the composition of infiltrated immune cells into a tumor. To this end, naïve tumors between 150-550 mm3 were examined for infiltrated T cells and myeloid cells expressed as percentages of total CD45+ cells (Figure 4). The myeloid compartment across all tumors was evenly distributed across M2 macrophages, monocytic MDSCs, and granulocytic MDSCs. M1 macrophage infiltrate was comparatively lower than other myeloid derived cells and was slightly higher in EMT6-Luc tumors compared to EMT6 tumors. The infiltrate of CD4+ and CD8+ T cells was minimal yet consistent in both EMT6 and EMT6-Luc tumors with a slight elevation of NK cells in the EMT6-Luc tumors. This composition is suggestive of an immunosuppressive microenvironment which could help explain the limited response of the immunotherapy regimens tested.
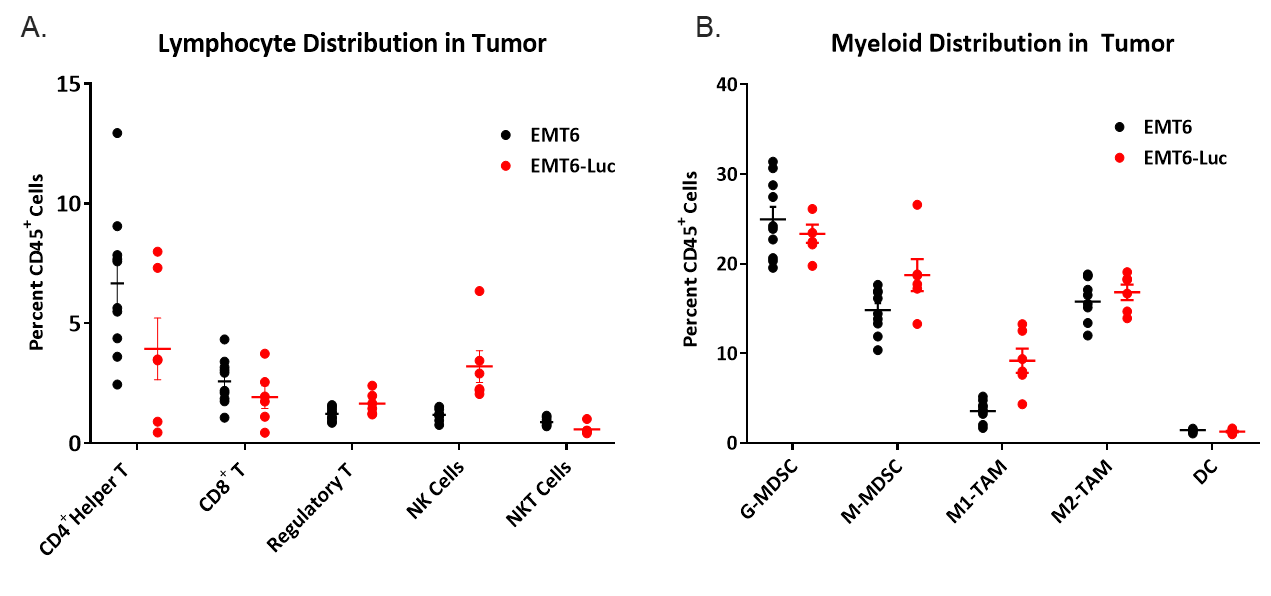
Figure 4. Lymphoid (A) and myeloid (B) immune cell distribution in naïve EMT6 and EMT6-Luc tumors.
The EMT6-Luc murine breast carcinoma model has a favorable immune profile that takes advantage of the complete mouse immune system and serves as a powerful tool in the immuno-oncology space. These data support use of the EMT6-Luc model as a robust preclinical immuno-oncology model for assessment of both primary tumor growth as well as formation of distant thoracic metastases.
Please contact us to speak with our scientists about how EMT6-Luc, or one of our other syngeneic models, can be used for your next immuno-oncology study.
References
1 ?American Cancer Society: Cancer Facts & Statistics.? American Cancer Society | Cancer Facts & Statistics, cancerstatisticscenter.cancer.org/?_ga=2.5394928.293340281.1606765768-996476487.1605734560.
2 Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
3Leal JHS and McArthur H. Breast cancer immunotherapy: From biology to current clinical applications. Eur. Med. J., 2020; 5(2): 113-124.
4Rockwell SC, Kallman, RF, Fajardo, LF. Characteristics of a Serially Transplanted Mouse Mammary Tumor and Its Tissue-Culture-Adapted Derivative. J. Natl. Can. Inst., 1972; 49(3): 735?749.


