Hepatocellular carcinoma (HCC) is an aggressive malignancy which has gradually increased in incidence to affect one million people worldwide and is currently the third leading cause of cancer related deaths in the world.[1,2] The prevalence of HCC is highest in Asia and Africa due to high endemicity of hepatitis B and C viruses which strongly predispose to the development of chronic liver disease and subsequent HCC.[1]
In the Western population, hepatitis C, alcoholic cirrhosis, and non-alcoholic steatohepatitis (NASH) are the main underlying causes. While early stage HCC treatment options include surgical excision, liver transplantation, chemo/radio embolization and radiofrequency ablation, the standard of care for advanced HCC is targeted therapies (sorafenib and regorafenib), radiation and chemotherapy (doxorubicin, 5-FU, and cisplatin).[3] Many clinical trials are underway for unresectable or advanced stage HCC with mono or combination immunotherapy, monoclonal antibodies or oncolytic virus therapy.[1-4] These therapies have demonstrated tumor shrinkage and improved survival but are not curative with the treatment outcome contingent on pre-existing conditions such as hepatitis, liver cirrhosis, or nonalcoholic fatty liver disease (NAFLD). Hence, there is an ever-increasing need for better treatment options. To evaluate novel therapies in a preclinical platform, Labcorp has established the syngeneic HCC model Hepa 1-6.
Hepa 1-6 is a murine hepatoma derived from the BW7756 hepatoma tumor that arose spontaneously in C57L/J mice, which contrasts with most available hepatoma models (BNL, A.7R.1, MH-129, MH134 and MH-22A) that are chemically transformed or induced lines. The Hepa 1-6 tumor model established in immunocompetent mice represents a clinically relevant model for preclinical testing of immunotherapy.
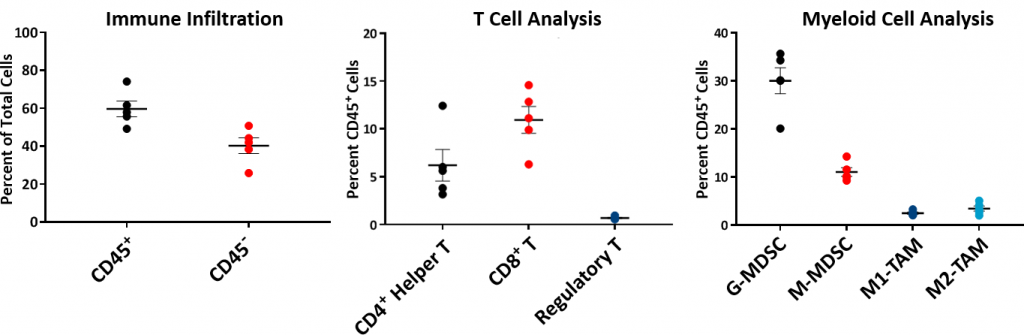
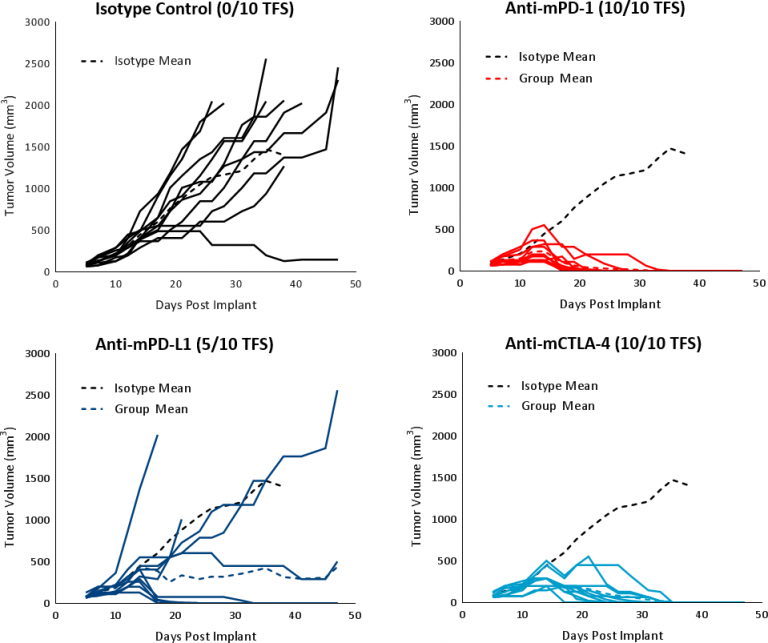
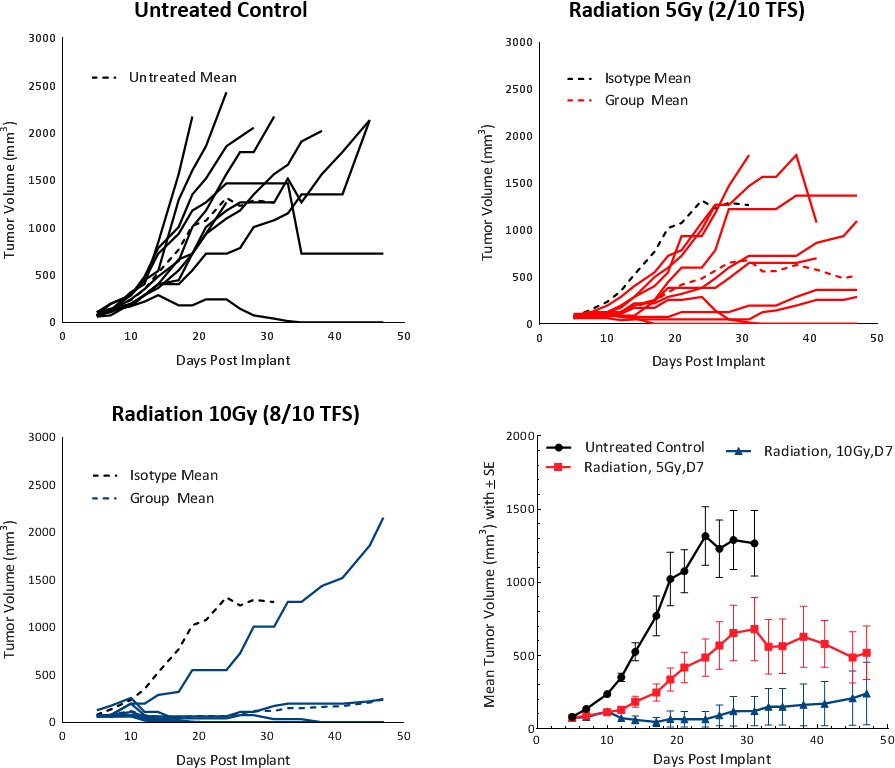
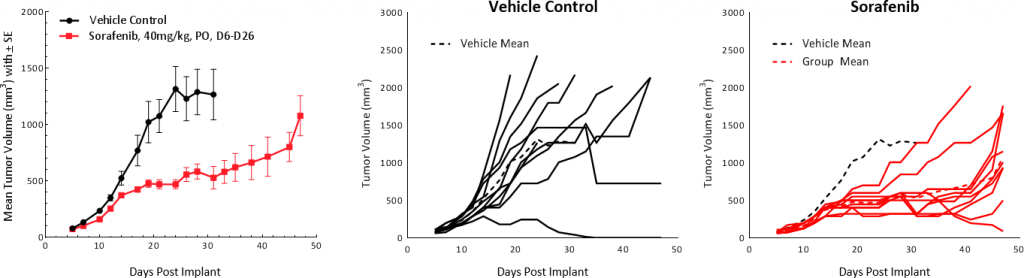
In this model spotlight we present in vivo data on Hepa 1-6 tumor model growth kinetics; along with response to checkpoint inhibitors alone or in combination with focal radiation. We tested the model in its parental C57L/J strain (limited availability at The Jackson Laboratories, Bar Harbor, ME) and the more readily available, histocompatable C57BL/6 strain.
Hepa 1-6 Growth Parameters
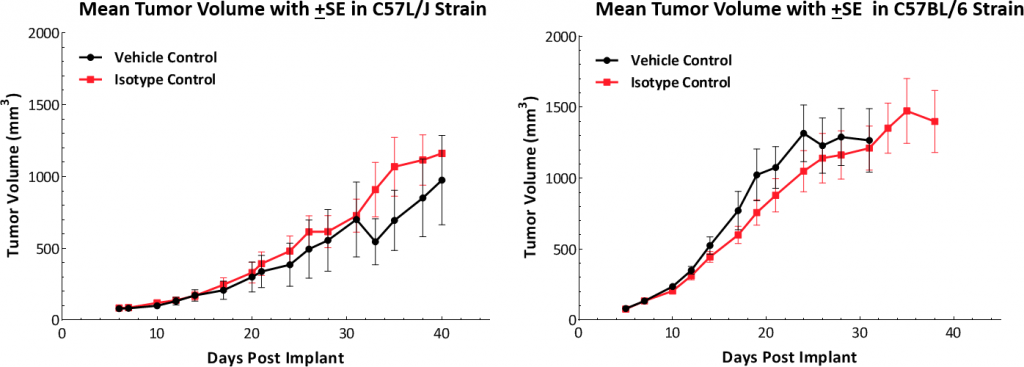
Growth kinetics following subcutaneous implant show that the model successfully establishes in both mouse strains (Fig. 1). The tumors have a median doubling time of ~5-6 days with no adverse body weight changes (data not shown). This steady growth rate allows for a three-week therapeutic window to evaluate anti-tumor responses.