Poster
Measuring human cytokines in mouse samples in the discovery setting: Understanding immunoassay technology and managing the risk
Introduction
At the protein level, humans and mice have similar, and at times nearly identical, amino acid sequences (Table 1). When human tumor and human lymphoid cells are used for immuno-oncology or chimeric antigen receptor T (CAR T) cell studies in immunocompromised mice, a range of methods can be used to assess their function and tumor regression. Here we discuss the technical aspects associated with immunoassays when considering the measurement of human proteins in a sample taken from a mouse. Understanding the immunoassay technology is essential when interpreting data from this unusual scenario.
ELISA
In the preclinical stage, the majority of laboratories purchase immunoassay methods from trusted commercial vendors to quantify the levels of their proteins of interest in serum or plasma. Contract Research Organizations (CROs) have a great deal of experience using commercially sourced immunoassays and can help with the decision-making process around the most appropriate method to use and which company to purchase from. These commercially sourced methods are typically sandwich enzyme-linked immunosorbent assay (ELISA) kits. In the preclinical discovery stage of drug development, a Good Laboratory Practice (GLP) validation of an immunoassay method is not needed, but quality data are certainly required. A GLP validation of an immunoassay to support the safety evaluation of a drug candidate will come later in the drug discovery and development process.
The ELISA method uses an antibody passively adsorbed to a plastic surface (usually a 96-well plate) to capture the target protein of interest. After a wash with buffer the sample is added. Following a short incubation period, the sample is washed away and another antibody (the ?detection? antibody) is added. The detection antibody binds to the captured target protein, forming the ?sandwich? (Figure 1). The detection antibody is usually tagged with an enzyme that will change a substrate color, or with a molecule that will give off light. The signal (optical density or light energy) is proportional to protein concentration in the sample. More detail on immunoassay technology can be found in numerous reviews (2?4).
The MSD Platform
The Meso Scale Discovery (MSD) platform is a versatile immunoassay platform. The assays that MSD market are sandwich immunoassays (ELISA) that emit light energy by electrochemiluminescence (ECL). MSD offers hundreds of immunoassay kits including countless multiplexing options for human, mouse and rat proteins that are commonly used by CROs, pharmaceutical and biotechnology companies, some academic institutions. MSD is the go-to platform for immunogenicity assays (also known as anti-drug antibody [ADA] assays) and is commonly used for custom-built pharmacokinetic (PK) assays to measure the concentration of a biologic drug. ADA and PK assays built on the MSD platform routinely pass the rigors of a GLP validation.
The MSD platform allows ?multiplexing?. Multiplexing is a technique that allows multiple analytes to be quantitated within a single well. Multiplexing saves time by cutting down on the number of plates that need to be processed and saves on sample amounts since the same 50 microliter sample can be analyzed up to ten different ways. Multiplexing is invaluable when assessing toxicity, determining the mechanism of action (MOA), or for biomarker discovery assays (5).
Matrix
The biological fluid added to the wells of the ELISA plate is the matrix. The matrix is commonly serum or plasma but can also be tissue culture media, vitreous humor (fluid from inside the eye), synovial fluid (joints), urine, tears, sweat or even cell lysis buffer used to release intracellular proteins. A matrix is a complex fluid and can cause a number of problems in an ELISA assay. Collectively, these problems are commonly referred to as the ?matrix effect?. The matrix effect is caused by interfering endogenous components, including albumin, fibrinogen, immunoglobulins, complement C4, apolipoproteins and haptoglobin, that differ in abundance and amino acid sequence between species. Matrix effects are typically investigated by dilution, dilutional linearity or spike and recovery experiments (6). The manufactures typically will claim that their immunoassay is validated for serum and/or plasma but not all the possible matrices. Commercially available immunoassays are rarely validated for serum and plasma for both human and mouse. Using an immunoassay for a purpose other than that intended by the manufacturer is an identifiable risk. Some manufacturer?s put disclaimers in their literature stating that the use of a matrix other than those they have validated will not be supported. Understanding the risk is important, especially when using a commercial assay for human protein quantitation from a mouse matrix.
Immunogen
Immunoassays use antibodies as tools to capture and detect proteins of interest (Figure 1). The immunogen is injected into animals to elicit an immune response. Antibodies derived from animals injected with the immunogen are used as critical materials in immunoassays. In the case of generating antibody reagents to be used in immunoassay development for a protein target, a protein is the immunogen. Protein immunogens of various sizes and from a variety of sources can be used. Recombinant proteins are commonly used, purified endogenous proteins are used less frequently. A laboratory-synthesized small peptide (8?20 amino acids), or a slightly larger peptide (20?100 amino acids), is routinely used because of the ease of synthesis of the peptide and of the known and relatively short amino acid sequence compared with the full-length protein. The amino-acid sequence of the immunogen is critically important information because of potential homology between species. A number of web-based resources containing amino acid sequence information about mouse and human proteins can be found in Table 2. Obtaining information about the immunogen used to generate an antibody product found in a commercially purchased ELISA assay may be difficult. Antibody and ELISA kit vendors often claim specific information about the amino acid sequence used to immunize the animal is a ?trade secret? or ?confidential?. A common entity in the antibody and ELISA kit business is the ?OEM? (Original Equipment Manufacturer) that many people associate with the automotive parts business. Many, but not all, companies that sell ELISA kits or antibodies do not actually make the kit or antibody themselves (7). They purchase the antibody or ELISA from someone else and then market it under their own branding. This creates issues for the consumer, including the availability of technical support assistance, information on timelines regarding when the product will ship, and price.
Cross Reactivity
Antibodies used as capture or detection reagents in immunoassays generally bind to only one protein, the analyte of interest. On occasion, an immunoassay can detect more than one protein. This is known as cross reactivity. The circumstance for measuring human cytokines in mouse matrix is very unusual. Species cross reactivity is where an immunoassay would detect a protein homolog from two or more species. Careful consideration of the amino acid sequence homology of the analyte between human and mouse is required to manage the potential of species cross reactivity. The amino acid sequence of the immunogen used to generate the capture and detection antibodies is critically important information; when using commercially purchased assays, the vendor will commonly not disclose this information.
Testing Species Cross Reactivity
To assess the possibility that the individual assays within the human multiplex would detect mouse cytokines, we generated samples in vitro with high levels of mouse cytokines for IFN?, IL-10, IL-2, IL-6 and TNF-? and applied the samples to the human multiplex. Cryopreserved mouse splenocytes were thawed into complete medium (RPMI 1640, 10% FBS, 1X GlutaMAX, 10 mM HEPES, 1 mM sodium pyruvate, and 1X non-essential amino acids). Live cells were counted via absolute count on a CytoFLEX LS Flow Cytometry platform. The cells were seeded into a 6-well plate at a density of 1x106 cells/mL at a volume of 10 mL/well. The plate was incubated in a 37?, 5% CO2 humidified incubator for 5 hours. The splenocytes were stimulated overnight with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL ionomycin. Unstimulated cells from the same spleen were incubated in parallel to serve as a negative control. At the end of the overnight culture period, the media was harvested, cells were removed by centrifugation (300rcf for 8 minutes), and the supernatants were frozen and stored at -80?. The cytokines secreted by the mouse splenocytes were analyzed in the V-PLEX Proinflammatory Panel 1 Mouse (catalog number K15048D-1) and the results are shown in Table 3. The cytokines secreted by the mouse splenocytes were subsequently analyzed in the V-PLEX Proinflammatory Panel 1 Human (catalog number K15049D-1) and the results are shown in Table 4. For the evaluable cytokines (IFN?, IL-10, IL-2, IL-6 and TNF-?) no species cross reactivity was observed.
Summary
Measuring human cytokines from a mouse sample is an unusual situation from a bioanalytical perspective. Understanding the technical aspect of potential interference is important when interpreting data generated from an immunoassay used for purposes outside the scope of the manufacturer?s intent. Species cross reactivity does not appear to be an issue for evaluable cytokines (IFN?, IL-10, IL-2, IL-6 and TNF-?) as tested in the MSD V-PLEX Proinflammatory Panel. Following careful evaluation of the information available, the assay does appear fit for use. For a more detailed discussion around the evaluation of human cytokine levels from mice that host human immune cells, please contact us using the link below.
Contact the preclinical oncology scientific team to learn more.
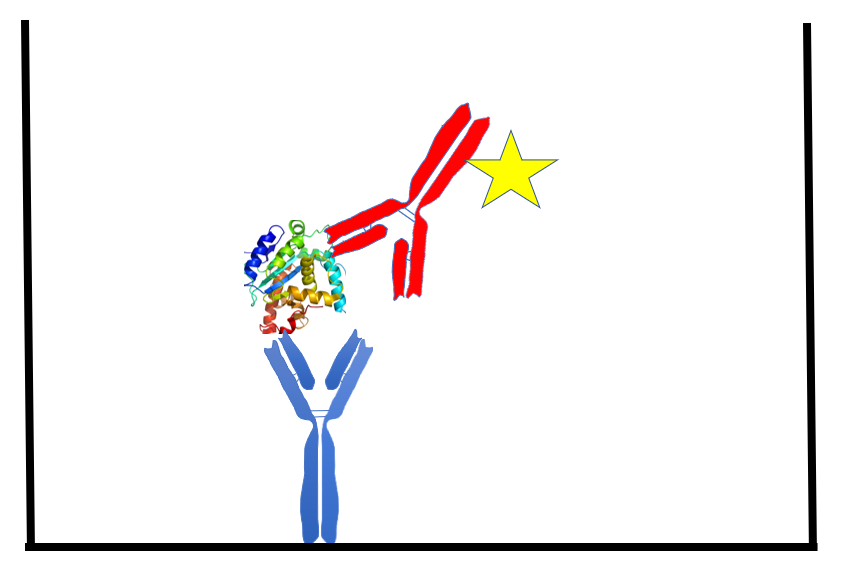
Figure 1. The sandwich ELISA. A mouse monoclonal antibody (blue), passively bound to the well of a 96-well plate, captures a protein analyte (target protein). A sulfo-tag (yellow star) conjugated rabbit polyclonal antibody (red) binds the captured analyte, forming a ?sandwich?. The interaction of the sulfo-tag and a substrate results in light that is measured by a camera within the MSD instrument. The amount of light generated is proportional to the amount of analyte captured.
Table 2. Selected web-based resources for protein and amino acid sequence information (8).
Table 3. Mouse splenocytes in cell culture were treated with PMA (phorbol 12-myristate 13 acetate) and ionomycin and the cell culture media was analyzed for mouse cytokines in the MSD V-PLEX Proinflammatory Panel 1 Mouse Kit. Values reported in pg/mL.
*BLD: below the limit of detection
References
1. Bult CJ, Krupke DM, Begley DA, et al. Mouse Tumor Biology (MTB): a database of mouse models for human cancer. Nucleic Acids Res. 2015 Jan;43(Database issue):D818-24.
2. Schwartz MK. Immunoassay of enzymes ? an overview. Clin Biochem. 1983;16(1):4?9.
3. Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015;72:4?15.
4. Porstmann T and ST Kiessing. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150(1?2):5?21.
5. Jani D, Allinson J, Berisha F, et al. Recommendations for use and fit-for-purpose validation of biomarker multiplex ligand binding assays in drug development. AAPS J. 2016;18(1):1?14.
6. Tu J, Bennett P. Parallelism experiments to evaluate matrix effects, selectivity and sensitivity in ligand-binding assay method development: pros and cons. Bioanalysis. 2017;9(14):1107?22.
7. Cameron MJ. How the business practices of your antibody vendor could impact your ligand-binding assay. Bioanalysis. 2014;6(12):1585?9.
8. Cowan KJ, Amaravadi L, Cameron MJ, et al. Recommendations for selection and characterization of protein biomarker assay calibrator material. AAPS J. 2017;19(6):1550?63.


