Hepatocellular carcinoma (HCC) affects over one million people globally and is currently the third leading cause of cancer related deaths in the world.[1] Worldwide, there are 800,000 new diagnoses and 700,000 patient deaths each year. The incidence of HCC is highest in Southeast Asia and Sub-Saharan Africa due to high prevalence of hepatitis B and C viruses which strongly predisposes one to develop chronic liver disease and subsequent HCC.[1,2] In the United States, there are expected to be 42,030 new diagnoses and 31,780 patient deaths in 2019.[2]
Depending on the stage of disease, HCC can be treated with surgery, chemotherapy, targeted therapies, and radiation.[4] Current clinical trials are tackling unresectable or advanced stage HCC with mono or combination immunotherapy, monoclonal antibodies or oncolytic virus therapy.[1-4] These approaches have demonstrated tumor shrinkage and improved survival, but are not curative. Hence, there is a need for improved treatment options. Labcorp has developed the syngeneic HCC model, Hepa 1-6, for preclinical evaluation of immunomodulatory drug candidates.
Hepa 1-6 is a murine hepatoma derived from the BW7756 hepatoma that arose spontaneously in C57L/J mice, which contrasts with most available hepatoma models (BNL, A.7R.1, MH-129, MH134 and MH-22A) that are chemically transformed or induced lines. Therefore, the Hepa 1-6 tumor model established in immunocompetent mice represents a clinically relevant model for preclinical testing of immunotherapy.
Review of Earlier Data
In our 2018 Hepa 1-6 Model Spotlight, we presented initial subcutaneous growth of the model and response data following dosing initiation with small but established tumors (mean tumor volume ~80-90 mm3). Treatment of Hepa 1-6 with checkpoint inhibitors in vivo was extremely effective against early stage disease, resulting in 50-100% durable complete responses, but leaving little room for improvement in combination with drug candidates. Throughout 2019, we have continued efforts to refine this model to enable checkpoint inhibition as a reasonable combination strategy with investigational drug candidates. Herein we present anti-tumor response data in a model of more advanced subcutaneous Hepa 1-6 disease.
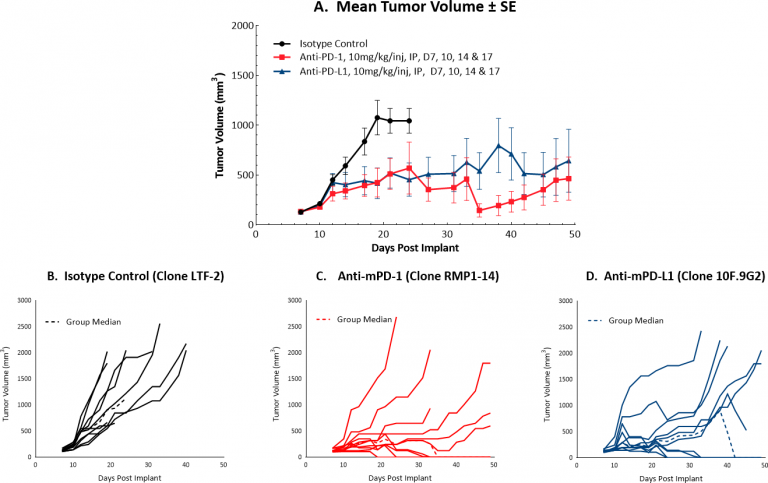
Checkpoint Blockade Response Data for Hepa 1-6
Initiating treatment with more advanced Hepa 1-6 tumors (at a mean tumor volume of ~130 mm3) resulted in a favorable response for testing drug candidates in combination with checkpoint blockade. Figure 1 illustrates mean (A) and individual (B-D) growth of control tumors and those treated with anti-mPD-1 or anti-mPD-L1 monotherapy. At this increased starting tumor volume, each monotherapy produced 40% durable complete responses, suggesting that while this strategy can certainly offer a means by which to test rational combination strategies, it is possible that the model can be further optimized. We will continue to refine this model to allow checkpoint blockade to maintain a moderate response, ideally with fewer complete responses. Small differences in mean tumor volume may mean large differences in response, so we are systematically approaching this challenge stepwise, with an ongoing study testing anti-tumor efficacy of checkpoint blockade with dosing initiation at a mean tumor volume of ~150 mm3. This study is not yet complete but is already indicating fewer complete responses compared to lower initiating tumor volumes (data not shown).