Poster
Preclinical oncology imaging capabilities and expertise
In this Tech Spotlight we will present an overview of some of the different non-invasive mouse imaging modalities we offer and the advantages of these services in biodistribution studies, monitoring of treatment efficacy and molecular target assessment of disease in tumor models. In the examples described below, animal care and use was conducted in an AAALAC-accredited facility in adherence with animal welfare regulations.
Positron Emission Tomography (PET)
PET is a type of nuclear medicine, functional imaging technique, which detects pairs of gamma rays emitted indirectly by positron-emitting radioisotope-labeled molecules (e.g., antibodies, antibody fragments, proteins, peptides, nanoparticles) and reconstructs their concentration within the body into 3D images.2,3 The most often used PET isotopes are 18F, 124I and 89Zr. 89Zr is frequently used as it possesses a physical half-life (t1/2=78.4 h) that is compatible with the in vivo pharmacokinetics of large molecules, particularly antibodies, and has been approved for clinical use. PET is used in preclinical studies to image tumors and metastasis, and understand disease biology, biodistribution and kinetics of biologic molecules along with metabolic activity within tumors. In the example below, the biodistribution and uptake of an 89Zr-tagged antigen (Ag) in Ag- and Ag+ tumors implanted subcutaneously (SC) in female NOD SCID mice was compared through the use of PET imaging. Once tumors were established, mice were injected intravenously (IV) with the 89Zr antigen. At different times after dosing, mice were anesthetized, placed one at a time in a dedicated Siemens Inveon D-PET small animal scanner and statically scanned. PET images were reconstructed using an iterative 3D ordered subsets expectation maximization (OSEM) algorithm. PET images were segmented with vendor-supplied software incorporated in the Amira image analysis package. As shown in Figure 1, PET scans clearly showed increased uptake in Ag+ tumors as compared to Ag- tumors, as well as differential uptake in tumors compared to liver and bone.
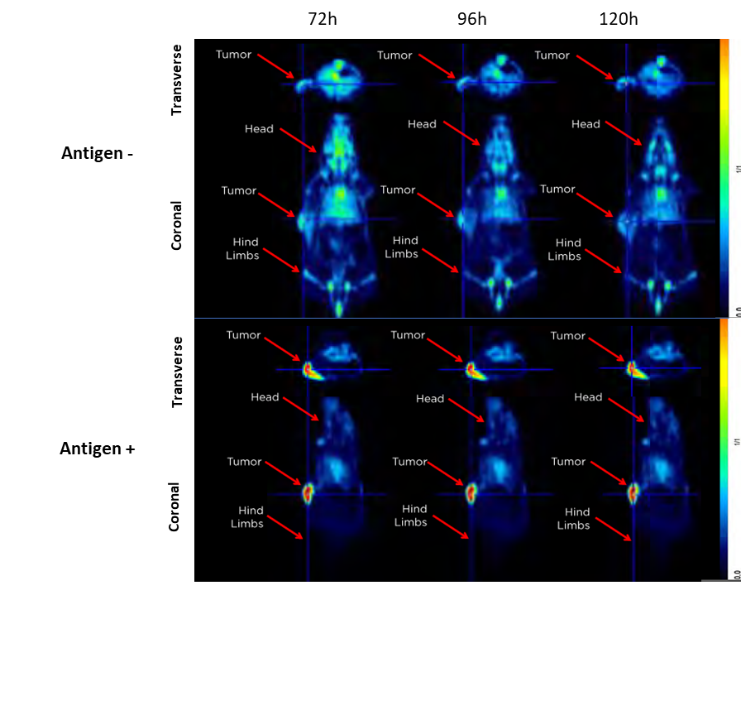
Figure 1. Representative image of Ag+ and Ag- tumors using PET after injection of an 89Zr labeled antigen. Transverse and coronal planes are shown.
ET imaging can also detect biochemical processes before anatomical changes occur. Indeed, the uptake of radiolabeled molecular probes can vary depending on the metabolic status of the tissue. One such radiopharmaceutical is 18F-Fludeoxyglucose (FDG), used for the assessment of abnormal glucose metabolism which occurs in cancer. In female nude mice implanted with colorectal carcinoma Colo-205 tumors, treatment led to a decrease in signal as shown in Figure 2A. Quantification of the FDG standardized uptake value (SUV) quantifying the degree of 18F-FDG accumulation within the tissue of interest, showed that 5 days post-treatment, tumors accumulated markedly less 18F-FDG and that difference was accentuated by day 8 post-treatment (Figure 2B).
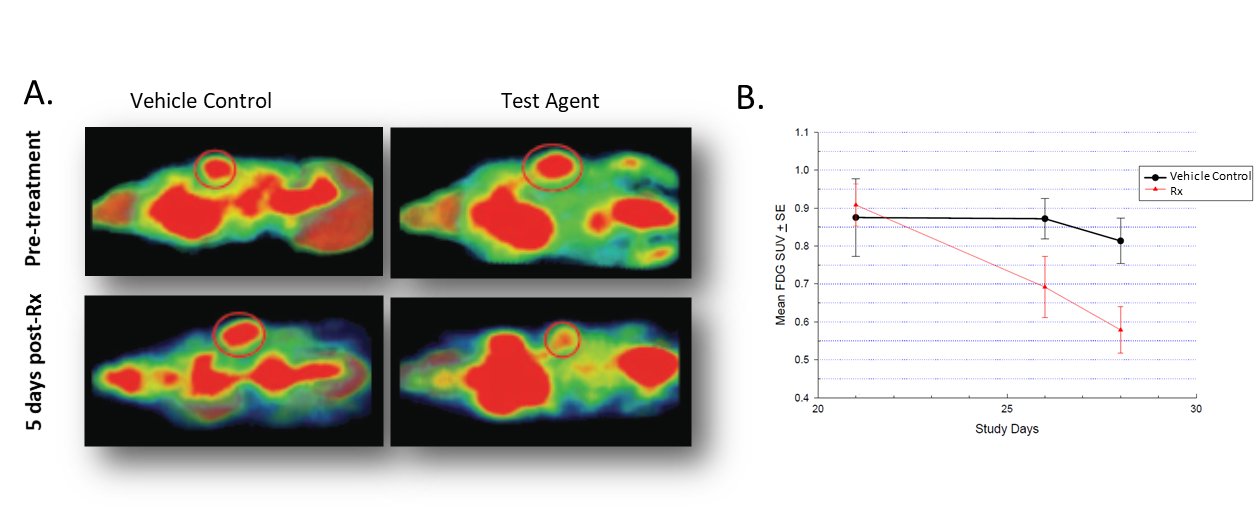
Micro-Computed Tomography (Micro-CT)
Micro-CT is a high-resolution (to ~15 microns) non-invasive imaging modality that generates true volumetric 3D images. The large density differences between bone, soft tissue and air make them readily distinguishable by CT and the use of systemically administered CT contrast agents enables better visualization of the soft tissues, including the vascular system. Used in preclinical studies to provide high resolution anatomic information, longitudinal tumor imaging and response to treatment, CT is considered the gold standard for bone imaging.4 We currently enroll a Bruker SkyScan-1176 Micro-CT scanner that can image down to 9 µm pixel size, equipped with a software that enables reconstruction, data-viewing, volume and surface rendering and image analysis in 2D and 3D. Longitudinal studies performed in rat pups showed skeletal development over time in exquisite detail (Figure 3A) and growth of different bones was quantified over time (Figure 3B). The effect of radiation and isoflurane on skeletal development was evaluated and shown not to have a significant effect on the growth of the bones studied (Figure 3C). This data set underscores the value of micro-CT in longitudinal imaging and highlights its potential use in monitoring disease progression and severity in bone metastatic models.
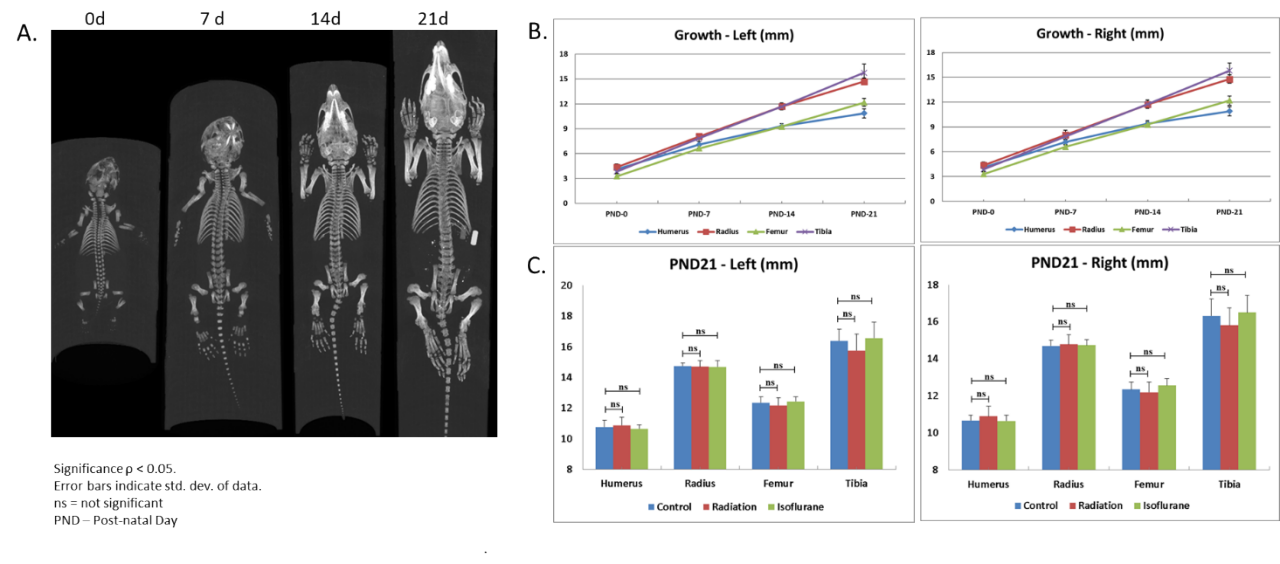
Figure 3. Rat skeletal development and effect of X-radiation and isoflurane exposure. A. Longitudinal imaging to monitor skeletal development. B. Quantification of bone growth. C. Effect of radiation and isoflurane.
Tumor development can also be monitored and quantified in preclinical tumor models using micro-CT. This modality is particularly valuable when assessing tumors without the use of specific radiolabeled or near-infrared labeled probes or luciferase-enabled tumor cell lines. Because the micro-CT system we use synchronizes images that are obtained from the breathing cycle and the cardiac cycle, this modality is particularly adapted to monitoring lung cancer. In the example shown in Figure 4, human non-small cell lung adenocarcinoma H441 was implanted OT in female nude mice and the effect of the standard of care gemcitabine for lung cancer was evaluated. Tumors developed over time, as shown in Figure 4 A (arrows), and gemcitabine treatment inhibited tumor growth. Quantification of tumor volume clearly showed gemcitabine, at both doses tested, significantly prevented tumor growth (Figure 4B).
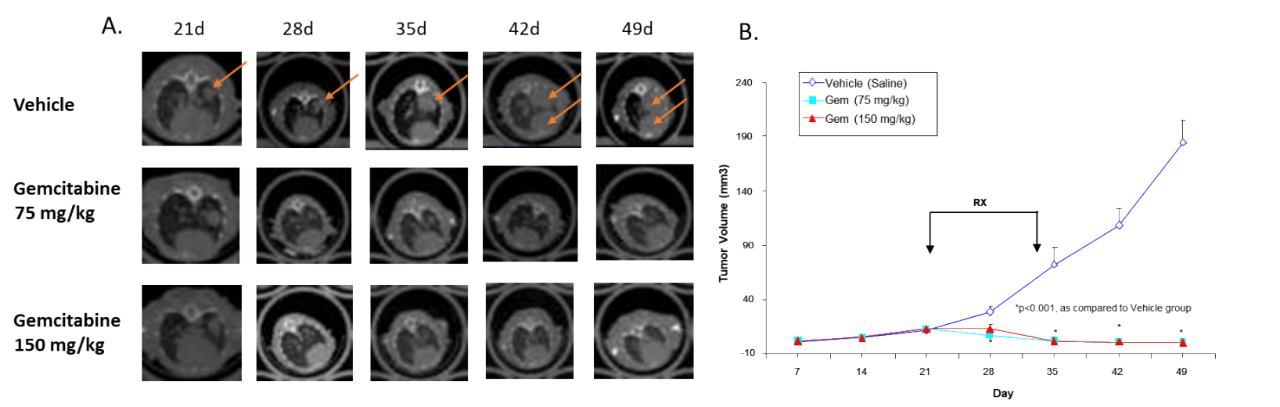
Figure 4: Effect of gemcitabine against human non-small cell lung adenocarcinoma H441 implanted OT into nude mice. A. Representative longitudinal images. B. Quantification of tumor volume.
Micro-CT provides high resolution, 3-dimensional, rapid data acquisition and sensitivity to both skeletal and soft tissue. While it has been used primarily for bone imaging, micro-CT is also effective in longitudinal imaging of tumor development and response to therapeutic interventions, visualization of blood vessels, angiogenesis, ectopic calcification, and in toxicology studies.
Additional information on MRI and PET imaging can be found here.
Bioluminescence Imaging (BLI)
BLI is used to non-invasively visualize the spatiotemporal biodistribution of luciferase (luc)-enabled tumors and measure anti-tumor response to a variety of treatments, critical when screening for new therapies.5 Temporal imaging of bioluminescent signal and effect of therapies is exemplified in Figure 5. Mice were implanted intracranially with mouse glioma GL261-luc cells and imaged at different times post-implantation. Treatment with the checkpoint inhibitor anti-mPD-1 increased time on study slightly, while focal radiation had a much larger impact on tumor burden. The combination of focal radiation to the brain and systemic anti-mPD-1 had an even larger impact on tumor burden and time on study.
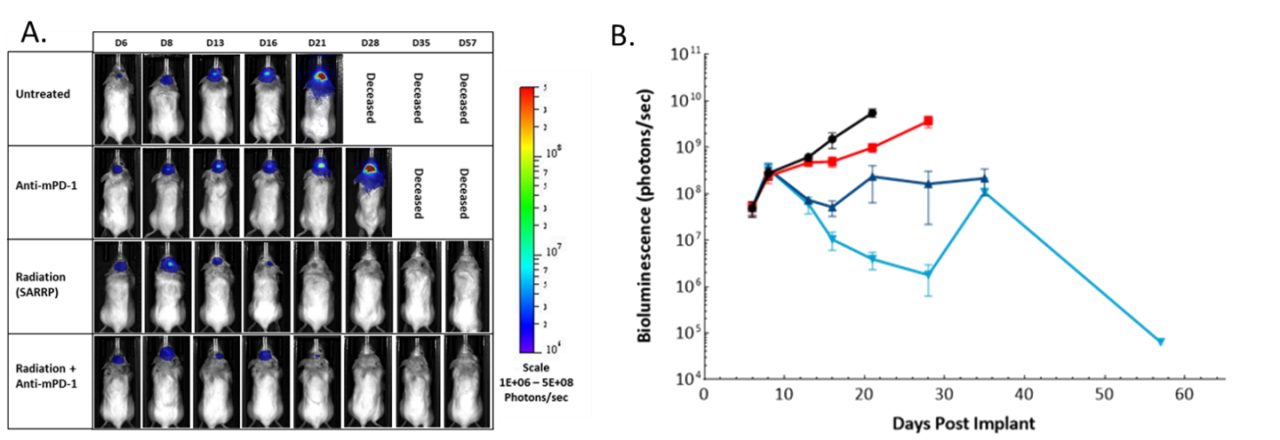
Figure 5: Effect of focal radiation, anti-mPD-1 and combination therapy in intracranial GL261-luc tumors in female C57BL/6 albino mice. A. Representative images. B. Quantification of BLI signal (photons/sec).
A decisive application for BLI is in tracking the effect of adoptive cell therapies in disseminated malignancies. Preclinical in vivo assessment of the efficacy and safety of chimeric antigen receptor (CAR-T) cells is vital before translating these new therapies into the clinic. The study shown in Figure 6 was designed to test different CAR-T preparations and doses in human Raji-luc B cell lymphoma cells implanted IV in NSG mice. The 3 different CAR-T constructs significantly inhibited tumor burden and prolonged survival, highlighting the value BLI brings to the development of these new cellular immunotherapies.
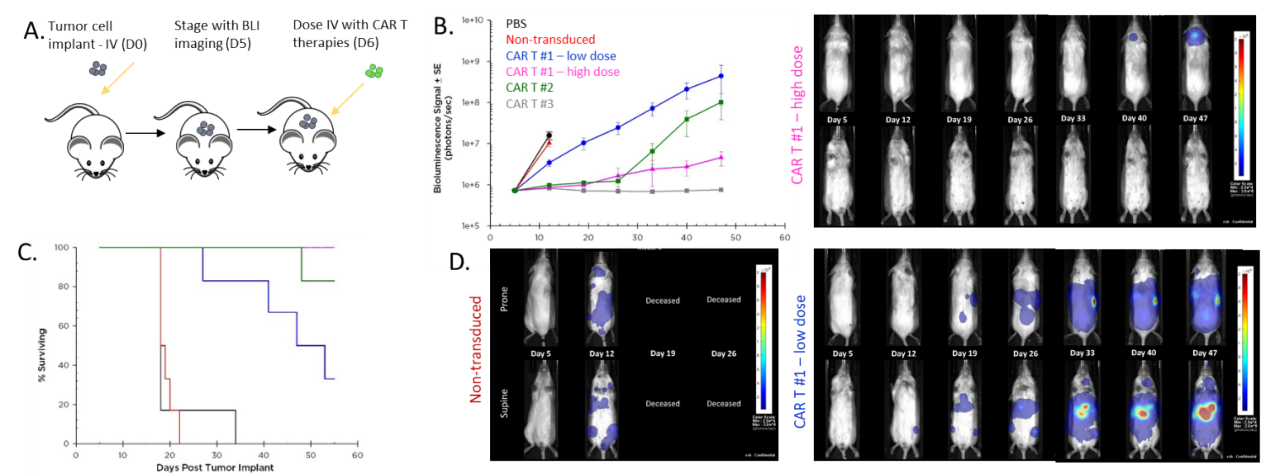
Figure 6: Effect of CAR-T therapy against human Raji-luc B cell lymphoma implanted IV into NSG mice. A. Schematic diagram of tumor cell inoculation and CAR-T therapy. B. Tumor burden assessed by BLI. C. Overall survival. D. Representative images.
For more details on BLI please see the Bioluminescence Imaging Technology Spotlight.
Fluorescence imaging (FLI)
FLI is a non-invasive optical imaging modality designed to visualize and quantify fluorescent signal in tissues. FLI offers a robust, sensitive and higher throughput alternative relative to radioactive imaging platforms or technologies requiring cells to be manipulated to express luciferase. As such, it could emerge as a clinical option in the detection of cancer in patients.6 It can be used in drug discovery to follow the biodistribution of fluorescently-labeled biologics or small molecules, effect of treatments on biological pathways, biomarker coverage and imaging of tumors which do not express luciferase. For FLI, animals are injected (injection route depending on the probe or test article) with near infrared fluorescence (NIRF)-tagged probes or test articles which can be passive circulating agents, targeted to specific cellular ligands or activated by particular biologic processes. The choice for fluorescence imaging in the NIRF spectrum (600-900 nm) allows to maximize tissue penetration and minimize absorption by physiologically abundant absorbers such as hemoglobin (< 600 nm) or water (> 900 nm). Mice are anesthetized and placed in an IVIS® system (PerkinElmer, MA). Collected fluorescence data are reconstructed using the Living Image® software and the signal (in photons/sec) calculated within regions of interest drawn encompassing the whole body, tumor or target tissue. FLI and NIRF imaging agents allow the characterization of key biochemical processes within a tumor, essential in monitoring tumor progression, staging, designing of new treatments and altering treatment options. FLI also allows imaging different biological pathways simultaneously using probes that are labeled with different fluorophores which absorb and emit at non-overlapping wavelengths. One such example (Figure 7) shows mice implanted with a foam polyether sponge soaked in either PBS or Complete Freund?s Adjuvant (CFA), as a model for granuloma which can occur in tumors, and imaged 6 and 24h after being injected IV with Cat B Fast 680TM and MMPSense 750 FAST (PerkinElmer, MA). These probes are optically silent and only become fluorescent when cleaved by the targeted enzymes. The images clearly show increased cathepsin and matrix metalloproteinase enzymatic activities in mice implanted with a CFA-soaked sponge.
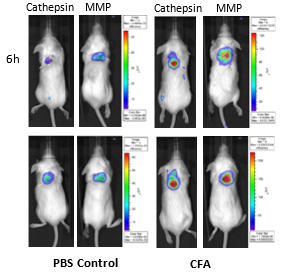
Figure 7: Imaging of BALB/c mice implanted SC with PBS and CFA-soaked sponges and injected IV with Cat B Fast 680TM and MMPSense 750 FAST.
In another example (Figure 8), FLI imaging shows the biodistribution of cathepsin-generated signal in mice implanted orthotopically (OT) with syngeneic mammary carcinoma 4T1-luc2-1A4 tumors and injected with a cathepsin-cleavable NIRF probe (ProSenseTM 750). FLI biodistribution assessment, by virtue of its safety and ease of use, may be ideal for the routine study, screening and improvement of small molecule imaging agents or larger therapeutic biomolecules.
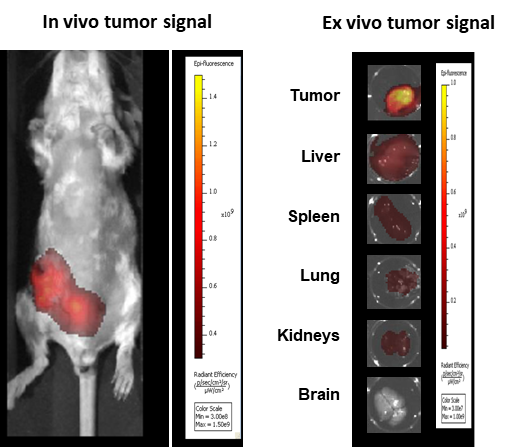
Figure 8: Imaging of BALB/c mice implanted OT with 4T1-luc2-1A4 and injected IV 24h before with ProSenseTM 750.
Multimodality
BLI can be coupled with FLI to interrogate different biological pathways. To illustrate this application, we used syngeneic 4T1-luc2-1A4 mouse breast adenocarcinoma cells injected OT into the mammary fat pad of immunologically competent BALB/c mice. When tumors reached ~300 mm3, ProSenseTM750 was injected IV to detect cathepsin activity associated with aggressive breast cancer growth. As shown in Figure 9, BLI and FLI tumor signals were readily detectable in tumors but not perfectly superimposable which is expected given that luciferase is produced by all living tumor cells while the fluorescent signal is confined not only to tumor cells but also other tumor-associated cells, mainly macrophages, responsible for the cleavage and uptake of the cathepsin ProSenseTM750 probe. Multimodality can thus be used to interrogate different biologies in vivo, simultaneously.
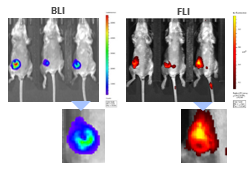
Figure 9: Multimodality imaging of 4T1-luc2-1A4 tumors by BLI (left) and FLI (right) 24h after IV injection of the cathepsin-specific ProSenseTM750 fluorescence probe. Below are closeups of the corresponding tumors.
Summary
Molecular imaging modalities, including MRI, PET, BLI and FLI, offer an invaluable alternative to end-stage endpoints like gross pathology, tissue weights, nodule counts, and/or histologic analysis for the assessment of tumor burden. By allowing longitudinal, real-time, non-invasive, in vivo assessment of tumor growth and burden, metastasis and response to therapeutic interventions, preclinical imaging promises improvements in the design and management of oncology models. For over a decade, we have offered a variety of industry-leading imaging modalities and enabling preclinical imaging tools and expertise to help support our clients for their drug discovery and development research. This Tech Spotlight reviews advantages these different imaging approaches offer, from visualizing morphological and structural changes to tracking the biodistribution of a molecule or understanding of a biologic pathway, among others.
Contact our scientists to request the full data set or to learn more about our preclinical oncology imaging services and how it can be applied to your preclinical research.
References
1 Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nature Reviews Drug Discovery, 2003; 2:123-131.
2 de Kemp RA, Epstein FH, Catana C, Tsui BMW, Ritman EL. Small-Animal Molecular Imaging Methods. Journal of Nuclear Medicine, 2010; 51 (S1): 18S-32S.3
3 Fiordelisi MF, Auletta L, MeomartinoL, Basso L, Fatone G, Salvatore M, Mancini M, Greco A. Preclinical Molecular Imaging for Precision Medicine in Breast Cancer Mouse Models. Molecular Imaging, 2019; 15:
4 Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. High Resolution X-ray Computed Tomography: An Emerging Tool for Small Animal Cancer Research. Neoplasia, 2000; 2(1-2): 62?70.
5 Alsawaftah N, Farooq A, Dhou S, Majdalawieh AF. Bioluminescence Imaging Applications in Cancer: A Comprehensive Review. IEEE Rev Biomed Eng , 2021; 14:307-326.
6 Ankersmit M, Bonjer HJ, Hannink G, Schoonmade LJ, van der Pas MHGM, Meijerink WJHJ. Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol, 2019; 23(12): 1113?1126.
Note: Please note that all animal care and use was conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.


