Poster
Subcutaneous and systemic preclinical modeling of A20 murine B cell lymphoma
Researchers use preclinical mouse modeling of lymphoma to explore novel combination strategies of conventional chemotherapy or radiation and immunomodulatory agents. To support these efforts, Labcorp has developed the murine B cell lymphoma model, A20. A20 was derived from a spontaneous reticulum cell sarcoma from an aging Balb/c mouse.[2] A20 tumors are reported to express PD-L1,[3] which is consistent with our RNAseq profile (data not shown) and concordant with several human lymphoma subtypes,[4] making it attractive for use in immunotherapy drug development.
In a 2017 Model Spotlight, we presented initial growth data as well as response to therapy initiated soon after a subcutaneous implant. In 2018, we continued efforts to expand characterization of this model. Herein we present initial anti-tumor response data in established subcutaneous A20 tumors as well as present growth kinetics of a systemic luciferase-enabled A20 model with tumor progression monitored by bioluminescence imaging (BLI).
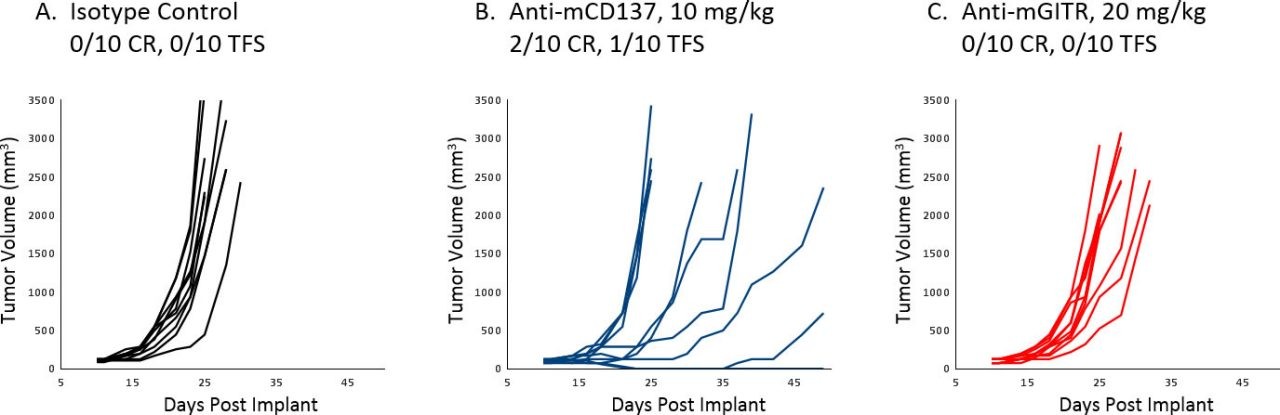
Treatment of A20 with immunotherapy agents in vivo is extremely effective against early stage disease, resulting in several complete responses. However, as expected, initiating treatment once tumors are established results in a much more limited response. Figure 1 illustrates individual (A-F) growth of control tumors and those treated with anti-mPD-1 or anti-mPD-L1 as monotherapy or in combination with focal radiation delivered by SARRP (XStrahl). Combination therapy produced a better than additive anti-tumor response, indicating that these strategies can be readily assessed in the A20 model once the tumors are established. Additionally, efficacy of anti-mGITR and anti-mCD137 against established A20 tumors was minimal to moderate, allowing for a broad dynamic range to assess improvement in combination of these antibodies and investigational agents (Figure 2 A-C).
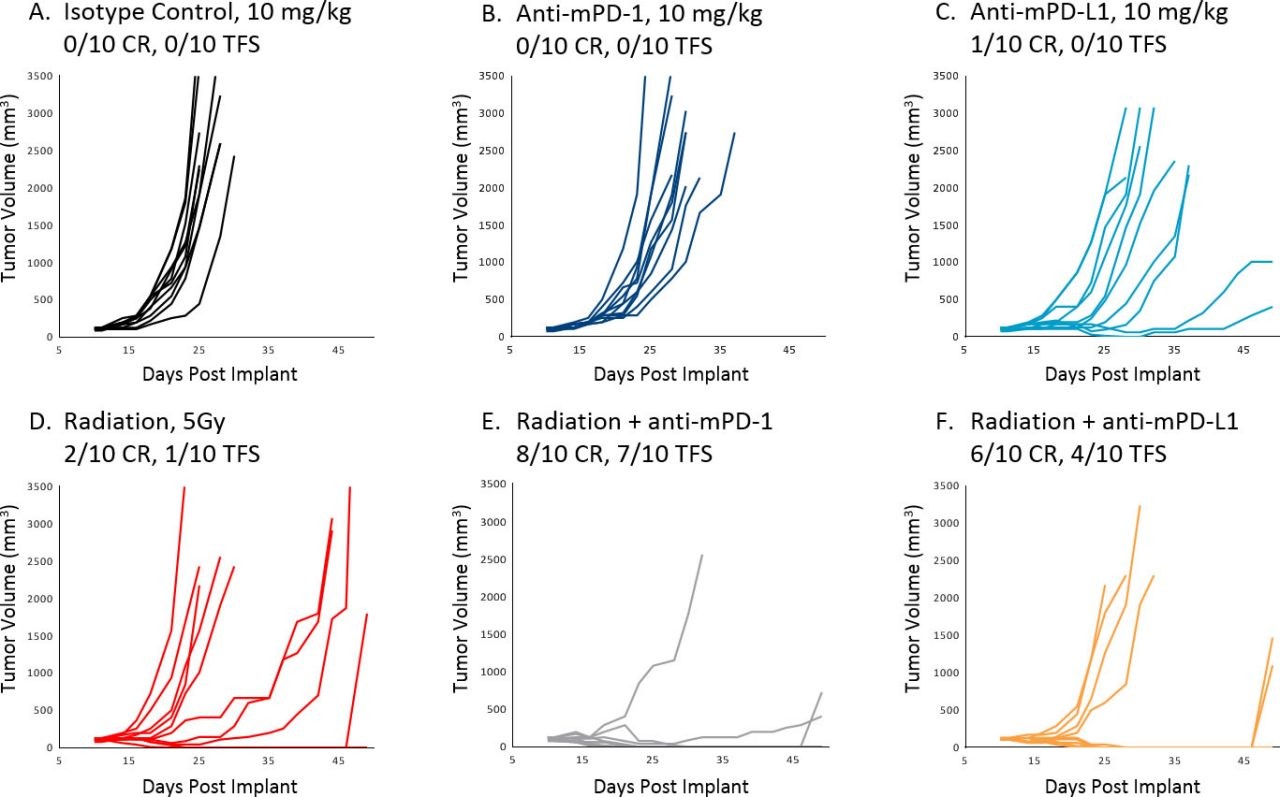
Fig. 1: Response of Established A20 Tumors to Treatment; CR=Complete Response, TFS=Tumor Free Survivor
To further increase utility of the A20 lymphoma model, we luciferase-enabled the A20 cell line which can be used to monitor tumor progression of systemic disease. The median doubling time of the model is 2.7 days with a therapeutic window of approximately three weeks.
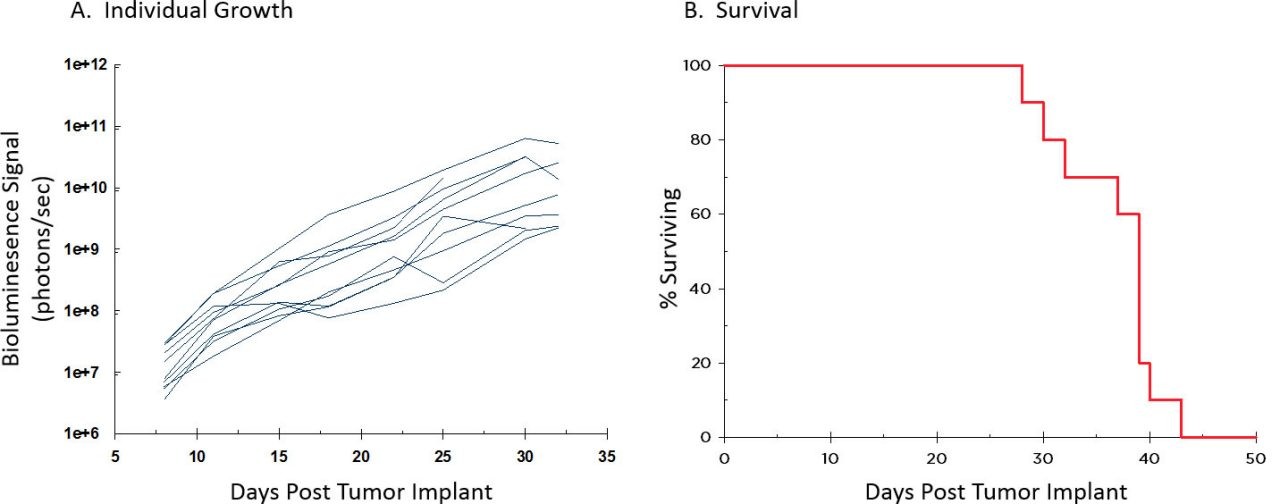
Fig. 3: Individual A20-Luc Growth Kinetics and Survival
Figure 3 shows individual tumor progression determined by BLI (A) and overall survival (B) which indicates favorable growth kinetics, and Figure 4 illustrates representative BLI imaging over time. Upon necropsy, a 100% incidence of liver lesions and 70% incidence of hindlimb paralysis and/or lesions on other organs (i.e., spleen, ovary, pancreas) were recorded. Abdominal distension was a common clinical sign manifesting between Days 30-32 and correlates well to the timing of strong BLI signal in Figures 3 and 4. A follow up study to investigate response to checkpoint blockade and costimulatory molecules is ongoing using this systemic model.
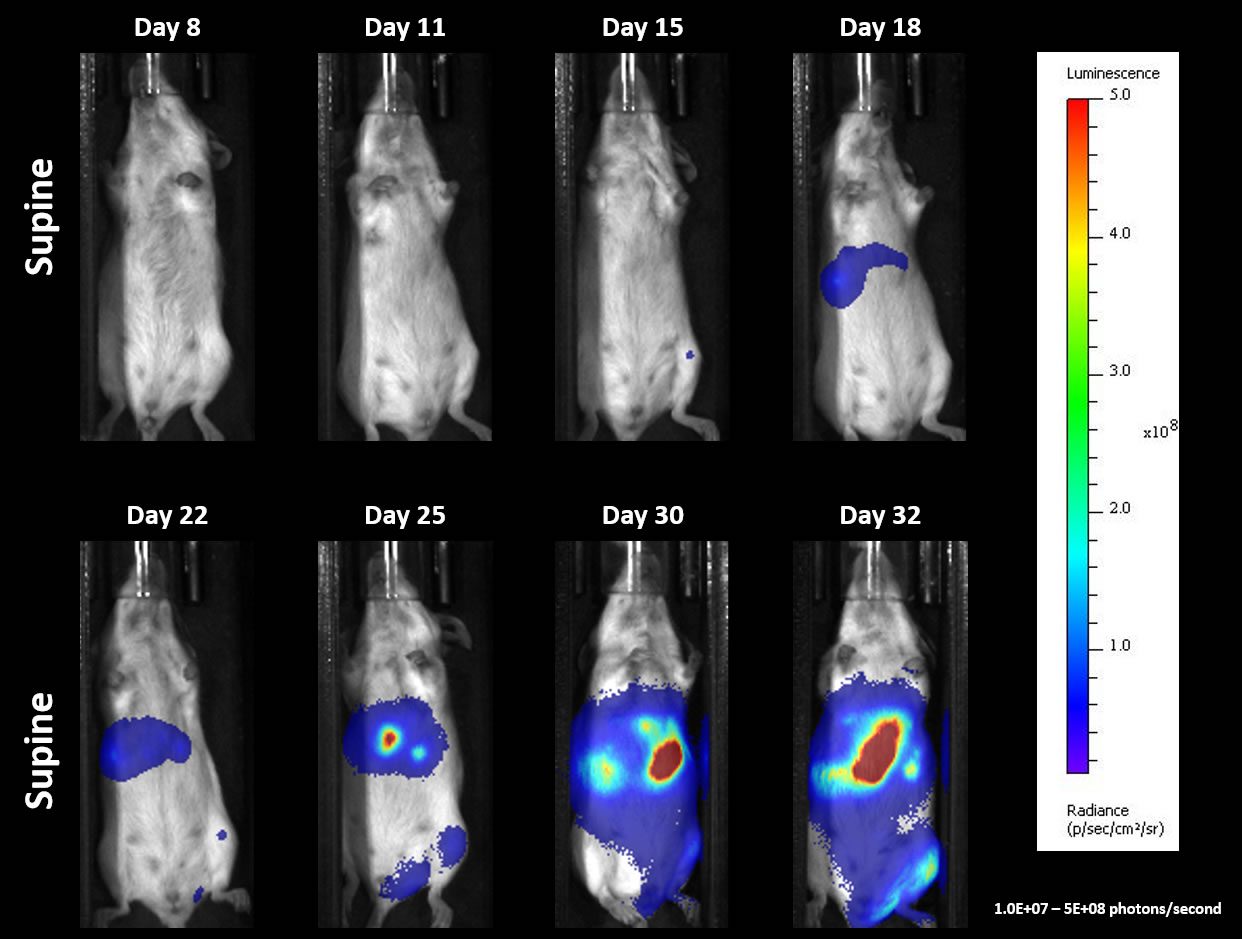
Fig. 4: Systemic A20-Luc ? Representative BLI Images Over Time
The A20 model provides robust preclinical means by which to study B cell lymphoma both by subcutaneous and systemic methods.
Please contact Labcorp to speak with our scientists about how A20 or one of our other syngeneic models can be used for your next immuno-oncology study.
References
[2] Kim KJ, Kanellopoulos Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. Journal of Immunology, 122(2): 549?554.
[3] Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, Ng PP, Chang BY, Levy L (2015) Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. PNAS, 112(9): E966-E972.


