Poster
Tumor models for pancreatic cancer
For the last 15 years, patients diagnosed with advanced stage pancreatic cancer are given gemcitabine (Gemzar®) as the standard first line treatment. Preclinically, we use gemcitabine as our standard of care to provide a benchmark to our clients looking to surpass current clinical treatment options or to combine with novel therapies; such as targeted agents and immune-modulators.
There are several human and murine pancreatic cell lines available to the preclinical cancer research community to aid in the development of novel therapies. Labcorp has a large panel of pancreatic lines ready for testing (see Table 1). We have optimized and characterized the subcutaneous (SC) growth for several of these models and evaluated their response to gemcitabine treatment.
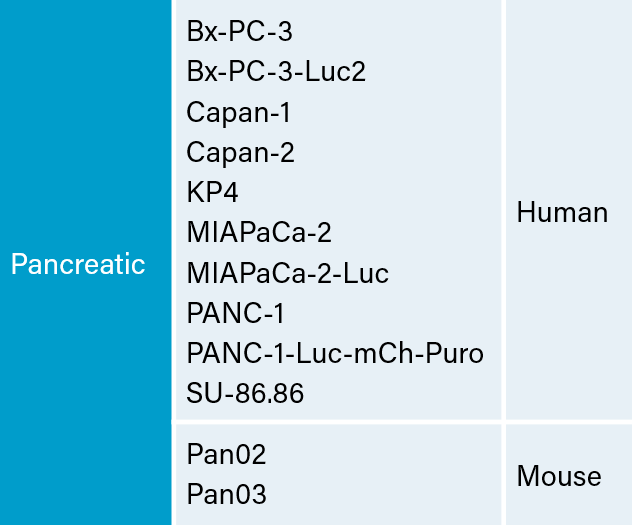
Pancreatic Carcinoma Cell Lines at Labcorp
PANC-1
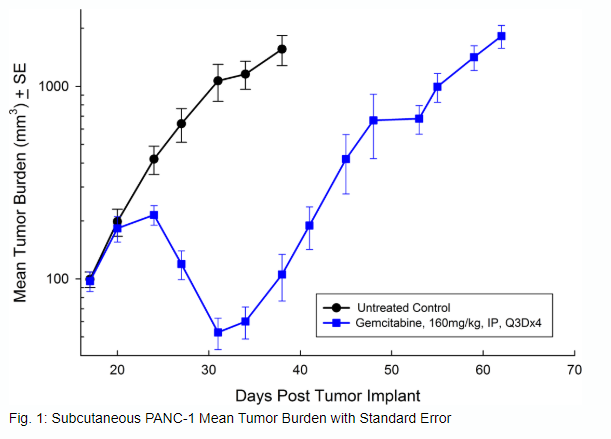
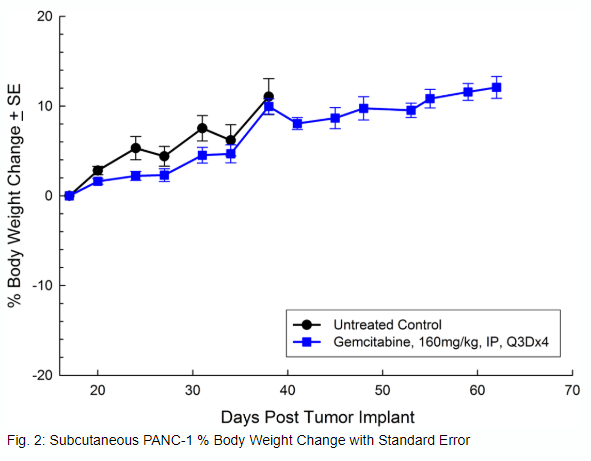
PANC-1 was isolated from a 56-year-old Caucasian man with pancreatic ductal adenocarcinoma. Subcutaneous tumor growth is reliable and consistent, with tumor volume doubling every 5 days and typically reaching evaluation size (~750mm3) in approximately 29 days post implant. Treatment with gemcitabine (160mg/kg) is well tolerated and produces statistically significant tumor growth delay, but is not curative (see Figures 1 and 2).
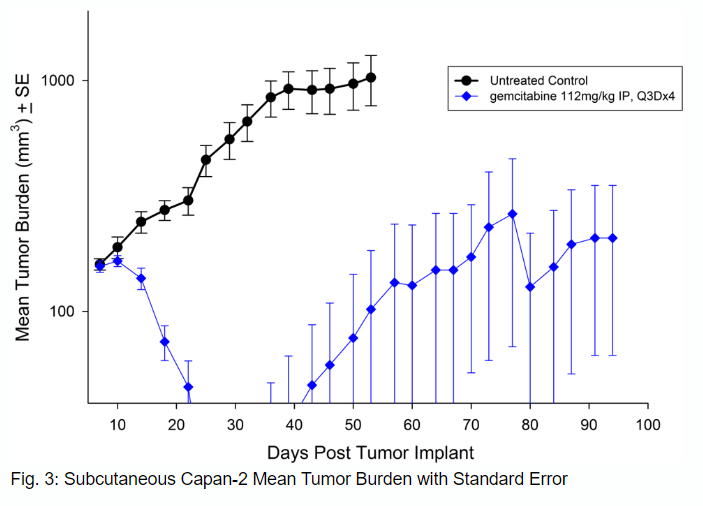
Capan-2
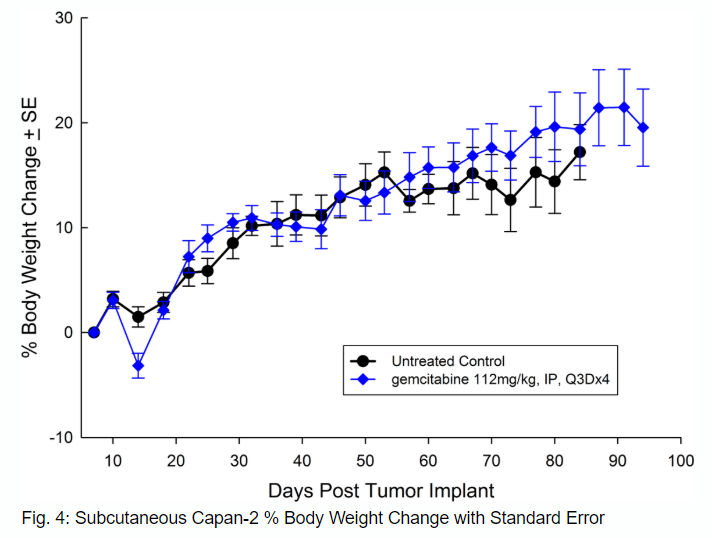
Capan-2, isolated from a 56-year-old Caucasian man with pancreatic ductal adenocarcinoma has subcutaneous tumor growth that is a bit slower than PANC-1 with a tumor volume doubling time of about 12 days and the time to reach evaluation size (750mm3) of about 36 days post implant. Treatment with gemcitabine (112mg/kg) is well tolerated and produces statistically significant tumor growth delay, with a large number of tumor free survivors (see Figures 3 and 4).
Bx-PC-3
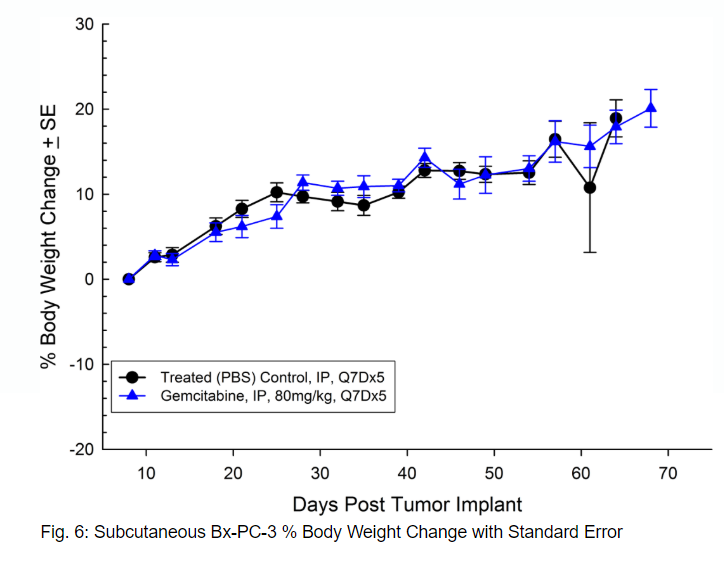
Bx-PC-3 was isolated from a 61-year-old Caucasian female with pancreatic adenocarcinoma. Subcutaneous tumor growth results in a tumor volume doubling time of about 16 days and the time to reach evaluation size (750mm3) of about 38 days post implant. Treatment with gemcitabine (80mg/kg) is well tolerated but fails to produce statistically significant tumor growth delay (see Figures 5 and 6).
MIAPaCa-2
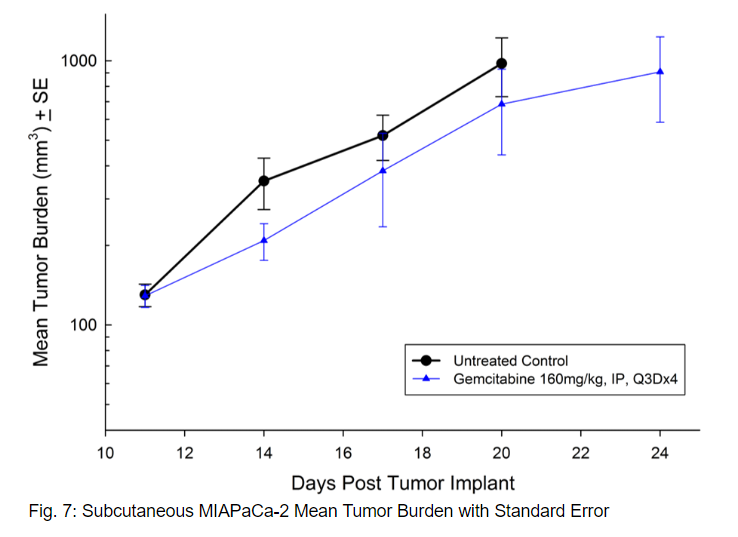
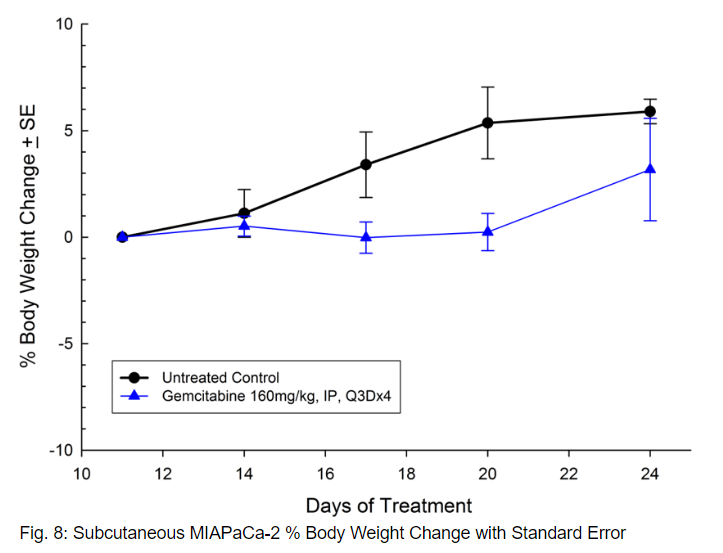
MIAPaCa-2 was isolated from a 65-year-old Caucasian man with pancreatic carcinoma. This model demonstrates very aggressive subcutaneous tumor growth with a tumor volume doubling time of about 3 days and the time to reach evaluation size (750mm3) of about 21 days post implant. Treatment with gemcitabine (160mg/kg) is well tolerated and produces statistically significant tumor growth delay, with one tumor free survivor observed in the study (see Figures 7 and 8).
As in the clinical response to standard of care, treatment response preclinically is highly dependent on the tumor line being used. There are many factors that can contribute to the responsiveness or lack thereof to gemcitabine treatment. However, looking across several different tumor lines within the same indication can provide key information about how effective a new therapy may be in the clinic.


